��Ŀ����
��֪��Ӧ��CO(g)+H2O(g)�� ![]() �� H2(g)+CO2(g)�� ��H=��41.2kJ/mol�����ɵ�CO2��H2�Բ�ͬ������Ȼ��ʱ�ں��������µķ�Ӧ���Ƶ�CH4��
�� H2(g)+CO2(g)�� ��H=��41.2kJ/mol�����ɵ�CO2��H2�Բ�ͬ������Ȼ��ʱ�ں��������µķ�Ӧ���Ƶ�CH4��
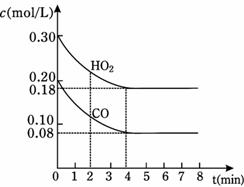
(1)850��ʱ��һ���Ϊ10 L�ĺ����ܱ������У�ͨ��һ������CO��H2O(g)��CO��H2O(g)Ũ�ȱ仯����ͼ��ʾ������˵����ȷ������������ (�����)��
A���ﵽƽ��ʱ����Ӧ��ϵ���ջ�ų�49.44kJ������
B����4minʱ����������ƽ����Է����������ٱ仯�����ж��Ѵﵽƽ�⣻
��C����6minʱ���������¶ȣ���Ӧƽ�ⳣ������������ 850��ʱ����Ũ�ȵı仯
D����8minʱ��������CO���ᵼ��v(��)��v(��)��ƽ��������Ӧ�����ƶ���
(2)850��ʱ�������ݻ�Ϊ2L���ܱ�������ͬʱ����1.0 mol CO��3.0 mol H2O��1.0 mol CO2��x mol H2����Ҫʹ������Ӧ��ʼʱ������Ӧ������У���xӦ����������������������� ��
(3)�罫H2��CO2��4��1������Ȼ�ϣ����ʵ��������¿��Ƶ�CH4����֪��
���� CH4(g) + 2O2(g)=CO2 (g)+ 2H2O (1)���� ��H1����890.3 kJ/mol
���� H2(g)+![]() O2(g)=H2O���������� (1)���� ��H2����285.8 kJ/mol
O2(g)=H2O���������� (1)���� ��H2����285.8 kJ/mol
��CO2(g)��H2(g)��Ӧ����CH4(g)��Һ̬ˮ���Ȼ�ѧ����ʽ�������������� ����������������
(4)������ȼ�ϵ����������̼����Ϊ����ʣ���CH4Ϊȼ�ϣ�����Ϊ��������ϡ����������Ϊ�缫��������ӦʽΪ������������������ ��������ӦʽΪ������������������������ ��
��1��A D ��2�֣� ��2��0��x��3�� x��3��2�֣�
��3��CO2(g) + 4H2(g) ![]() CH4 (g)+ 2H2O(l) ��H��-252.9 kJ/mol��2�֣�
CH4 (g)+ 2H2O(l) ��H��-252.9 kJ/mol��2�֣�
��4��CH4 +4CO32����8e��=5CO2+2H2O ��2�֣� 2O2+4CO2+8e-=4CO32- ��2�֣�

��8�֣���֪��Ӧ��CO(g) + H2O(g)H2(g) + CO2(g) ��H= Q kJ��mol-1����ƽ�ⳣ�����¶ȵı仯���±���
| �¶�/�� | 400 | 500 | 850 |
| ƽ�ⳣ�� | 9.94 | 9 | 1 |
��ش��������⣺
��1���������淴Ӧ��Q 0������ڡ���С�ڡ�����
��2��850��ʱ�����Ϊ10L��Ӧ���У�ͨ��һ������CO��H2O��g������������Ӧ��CO��H2O(g)Ũ�ȱ仯����ͼ���Իش�
��0��4 min��ƽ����Ӧ����v(CO)=______ mol/(L��min)
�����б�������Ϊ�ÿ��淴Ӧ�ﵽƽ���־����__________(�����)��
A�������������ܶȱ��ֲ���ʱ
B������Ӧ����0.1molCO(g)ͬʱҲ����0.1molH2O(g)ʱ
C����CO(g)�������������ֲ���ʱ
D�����÷�Ӧ�Ħ�H����ʱ
��3����Ҫ�ӿ�÷�Ӧ���ʣ�ͬʱ��Ҫ���CO��ת���ʣ��ɲ�ȡ�Ĵ�ʩΪ_____(�����)��
A������ˮ�������� B�������¶�
C��ʹ�ô��� D������ѹǿ E����������CO2
��8�֣���֪��Ӧ��CO(g) + H2O(g) H2(g) + CO2(g) ��H=" Q" kJ��mol-1����ƽ�ⳣ�����¶ȵı仯���±���
H2(g) + CO2(g) ��H=" Q" kJ��mol-1����ƽ�ⳣ�����¶ȵı仯���±���
| �¶�/�� | 400 | 500 | 850 |
| ƽ�ⳣ�� | 9.9 4 4 | 9 | 1 |
��1���������淴Ӧ��Q 0������ڡ���С�ڡ�����
��2��850��ʱ�����Ϊ10L��Ӧ���У�ͨ��һ������CO��H2O��g������������Ӧ��CO��H2O(g)Ũ�ȱ仯����ͼ���Իش�
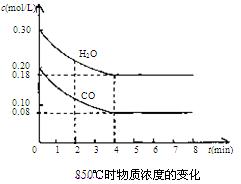
��0��4 min��ƽ����Ӧ����v(CO)="______" mol/(L��min)
�����б�������Ϊ�ÿ��淴Ӧ�ﵽƽ���־����__________(�����)��
A�������������ܶȱ��ֲ���ʱ
B������Ӧ����0.1molCO(g) ͬʱҲ����0.1molH2O(g)ʱ
C����CO(g)�������������ֲ���ʱ
D�����÷�Ӧ�Ħ�H����ʱ
��3����Ҫ�ӿ�÷�Ӧ���ʣ�ͬʱ��Ҫ���CO��ת���ʣ��ɲ�ȡ�Ĵ�ʩΪ_____(�����)��
A������ˮ�������� B�������¶�
C��ʹ�ô��� D������ѹǿ E����������CO2
��8�֣���֪��Ӧ��CO(g) + H2O(g) H2(g) + CO2(g) ��H= Q kJ��mol-1����ƽ�ⳣ�����¶ȵı仯���±���
|
�¶�/�� |
400 |
500 |
850 |
|
ƽ�ⳣ�� |
9.94 |
9 |
1 |
��ش��������⣺
��1���������淴Ӧ��Q 0������ڡ���С�ڡ�����
��2��850��ʱ�����Ϊ10L��Ӧ���У�ͨ��һ������CO��H2O��g������������Ӧ��CO��H2O(g)Ũ�ȱ仯����ͼ���Իش�

��0��4 min��ƽ����Ӧ����v(CO)=______ mol/(L��min)
�����б�������Ϊ�ÿ��淴Ӧ�ﵽƽ���־����__________(�����)��
A�������������ܶȱ��ֲ���ʱ
B������Ӧ����0.1molCO(g) ͬʱҲ����0.1molH2O(g)ʱ
C����CO(g)�������������ֲ���ʱ
D�����÷�Ӧ�Ħ�H����ʱ
��3����Ҫ�ӿ�÷�Ӧ���ʣ�ͬʱ��Ҫ���CO��ת���ʣ��ɲ�ȡ�Ĵ�ʩΪ_____(�����)��
A������ˮ�������� B�������¶�
C��ʹ�ô��� D������ѹǿ E����������CO2

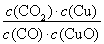
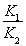
 H2(g)+CO2(g)
H2(g)+CO2(g)