��Ŀ����
�����仯�����ڹ�ũҵ�����о�����Ҫ���ã�
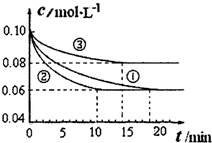
��1��ijС����й�ҵ�ϳɰ�N2��g��+3H2��g��?2NH3��g����H��0��ģ���о�����1L�ܱ������У��ֱ����0.1molN2��0.3molH2��ʵ��١��ڡ�����c��N2����ʱ�䣨t���ı仯��ͼ��ʾ��
ʵ��ڴӿ�ʼ���ﵽƽ��״̬�Ĺ����У���H2��ʾ��ƽ����Ӧ����Ϊ______��
��ʵ�����ȣ�ʵ��������õ�ʵ����������Ϊ______������ĸ����ʵ��������õ�ʵ����������Ϊ______������ĸ����
a������ѹǿb����Сѹǿc�������¶�d�������¶�e��ʹ�ô���
��2��NH3�����ڴ��������еĵ�������䷴Ӧԭ��Ϊ2NH3��g��+NO��g��+NO2��g��?2N2��g��+3H2O��g����H��O��
����߷����е��������ת���ʣ��ɲ�ȡ�Ĵ�ʩ��______������ĸ��
a�������¶�b������ѹǿc������NH3��Ũ��
��3��NCl3��ˮ����ˮ�ⷴӦ������NH3��ͬʱ�õ�______���ѧʽ����ClO2�ǹ���Ч���������Ʊ�ԭ��ΪNCl3+6ClO2-+3H2O=NH3��+6ClO2+3OH-+3Cl-����ת��1mol���ӣ�����ȡClO2������Ϊ______��
��4��25��ʱ����amol?L-1�İ�ˮ��bmol?L-1����������ϣ���Ӧ����Һǡ�������ԣ���a______b�����������������=��������a��b��ʾNH3?H2O�ĵ���ƽ�ⳣ��Ϊ______��
��1��ijС����й�ҵ�ϳɰ�N2��g��+3H2��g��?2NH3��g����H��0��ģ���о�����1L�ܱ������У��ֱ����0.1molN2��0.3molH2��ʵ��١��ڡ�����c��N2����ʱ�䣨t���ı仯��ͼ��ʾ��
ʵ��ڴӿ�ʼ���ﵽƽ��״̬�Ĺ����У���H2��ʾ��ƽ����Ӧ����Ϊ______��
��ʵ�����ȣ�ʵ��������õ�ʵ����������Ϊ______������ĸ����ʵ��������õ�ʵ����������Ϊ______������ĸ����
a������ѹǿb����Сѹǿc�������¶�d�������¶�e��ʹ�ô���
��2��NH3�����ڴ��������еĵ�������䷴Ӧԭ��Ϊ2NH3��g��+NO��g��+NO2��g��?2N2��g��+3H2O��g����H��O��
����߷����е��������ת���ʣ��ɲ�ȡ�Ĵ�ʩ��______������ĸ��
a�������¶�b������ѹǿc������NH3��Ũ��
��3��NCl3��ˮ����ˮ�ⷴӦ������NH3��ͬʱ�õ�______���ѧʽ����ClO2�ǹ���Ч���������Ʊ�ԭ��ΪNCl3+6ClO2-+3H2O=NH3��+6ClO2+3OH-+3Cl-����ת��1mol���ӣ�����ȡClO2������Ϊ______��
��4��25��ʱ����amol?L-1�İ�ˮ��bmol?L-1����������ϣ���Ӧ����Һǡ�������ԣ���a______b�����������������=��������a��b��ʾNH3?H2O�ĵ���ƽ�ⳣ��Ϊ______��

��1����ͼ��֪��ʵ���10min����ƽ�⣬ƽ��ʱ����N2��=0.1mol/L-0.06mol/L=0.04mol/L���ɷ���ʽ��֪����c��H2��=3����N2��=3��0.04mol/L=0.12mol/L����v��H2��=
=0.012mol/��L?min����
��ͼ��֪����ʵ�����ȣ�ʵ��ڵ���ƽ������ʱ��϶̣���Ӧ���ʽϿ죬��ƽ��ʱ������Ũ�Ȳ��䣬�ı�����ƽ�ⲻ�ƶ����÷�Ӧ����Ӧ�����������С�ķ�Ӧ������ѹǿƽ����ƶ�����ʵ���Ӧ��ʹ�ô�����
��ͼ��֪����ʵ�����ȣ�ʵ��۵���ƽ������ʱ��϶̣���Ӧ���ʽϿ죬ƽ��ʱ������Ũ�����ı�����ƽ�������ƶ����÷�Ӧ����Ӧ�������С�ķ��ȷ�Ӧ����Ϊ�����¶ȣ�
�ʴ�Ϊ��0.012mol/��L?min����e��c��
��2����߷����е��������ת���ʣ�Ӧ�ı�����ʹƽ��������Ӧ�ƶ���
a���÷�Ӧ����Ӧ�Ƿ��ȷ�Ӧ�������¶ȣ�ƽ�����淴Ӧ�ƶ������������ת���ʽ��ͣ���a����
b���÷�Ӧ����Ӧ���������ķ�Ӧ������ѹǿ��ƽ�����淴Ӧ�ƶ������������ת���ʽ��ͣ���b����
c������NH3��Ũ�ȣ�ƽ��������Ӧ�ƶ������������ת��������c��ȷ��
�ʴ�Ϊ��c��
��3��NCl3��ˮ����ˮ�ⷴӦ��NCl3�����и���ԭ�ӽ��ˮ����������ӣ�����ԭ�ӽ��ˮ�����ȥ������ӣ�������NH3��ͬʱ���õ�HClO��
��Ӧ��ֻ����Ԫ�صĻ��ϼ۷����仯����Ԫ����NCl3��+1����ΪCl-��-1�ۣ���ClO2-��+3������ΪClO2��+4�ۣ���ת��1mol���ӣ�����ClO2�����ʵ���Ϊ1mol������Ϊ1mol��67.5g/mol=67.5g��
�ʴ�Ϊ��HClO��67.5��
��4����Һ�����ԣ�����c��H+��=c��OH-������Һ�ĵ���غ�ɵã�c��H+��+c��NH4+��=c��Cl-��+c��OH-������c��NH4+��=c��Cl-�����Ȼ����ǿ����������ˮ��Һ�����ԣ�Ҫʹ�Ȼ����Һ�����ԣ���ˮӦ����������Ϊ����Ͱ�ˮ�������ȣ���ˮ�����ʵ���Ũ�ȴ������ᣬ
��Һ��c��H+��=c��OH-��=10-7mol/L��c��NH4+��=c��Cl-��=
mol/L��c��NH3?H2O��=��
-
��mol/L��
���볣��ֻ���¶��йأ����ʱNH3?H2O�ĵ��볣��Kb=
=
=
��
�ʴ�Ϊ������
��
| 0.12mol/L |
| 10min |
��ͼ��֪����ʵ�����ȣ�ʵ��ڵ���ƽ������ʱ��϶̣���Ӧ���ʽϿ죬��ƽ��ʱ������Ũ�Ȳ��䣬�ı�����ƽ�ⲻ�ƶ����÷�Ӧ����Ӧ�����������С�ķ�Ӧ������ѹǿƽ����ƶ�����ʵ���Ӧ��ʹ�ô�����
��ͼ��֪����ʵ�����ȣ�ʵ��۵���ƽ������ʱ��϶̣���Ӧ���ʽϿ죬ƽ��ʱ������Ũ�����ı�����ƽ�������ƶ����÷�Ӧ����Ӧ�������С�ķ��ȷ�Ӧ����Ϊ�����¶ȣ�
�ʴ�Ϊ��0.012mol/��L?min����e��c��
��2����߷����е��������ת���ʣ�Ӧ�ı�����ʹƽ��������Ӧ�ƶ���
a���÷�Ӧ����Ӧ�Ƿ��ȷ�Ӧ�������¶ȣ�ƽ�����淴Ӧ�ƶ������������ת���ʽ��ͣ���a����
b���÷�Ӧ����Ӧ���������ķ�Ӧ������ѹǿ��ƽ�����淴Ӧ�ƶ������������ת���ʽ��ͣ���b����
c������NH3��Ũ�ȣ�ƽ��������Ӧ�ƶ������������ת��������c��ȷ��
�ʴ�Ϊ��c��
��3��NCl3��ˮ����ˮ�ⷴӦ��NCl3�����и���ԭ�ӽ��ˮ����������ӣ�����ԭ�ӽ��ˮ�����ȥ������ӣ�������NH3��ͬʱ���õ�HClO��
��Ӧ��ֻ����Ԫ�صĻ��ϼ۷����仯����Ԫ����NCl3��+1����ΪCl-��-1�ۣ���ClO2-��+3������ΪClO2��+4�ۣ���ת��1mol���ӣ�����ClO2�����ʵ���Ϊ1mol������Ϊ1mol��67.5g/mol=67.5g��
�ʴ�Ϊ��HClO��67.5��
��4����Һ�����ԣ�����c��H+��=c��OH-������Һ�ĵ���غ�ɵã�c��H+��+c��NH4+��=c��Cl-��+c��OH-������c��NH4+��=c��Cl-�����Ȼ����ǿ����������ˮ��Һ�����ԣ�Ҫʹ�Ȼ����Һ�����ԣ���ˮӦ����������Ϊ����Ͱ�ˮ�������ȣ���ˮ�����ʵ���Ũ�ȴ������ᣬ
��Һ��c��H+��=c��OH-��=10-7mol/L��c��NH4+��=c��Cl-��=
| b |
| 2 |
| a |
| 2 |
| b |
| 2 |
���볣��ֻ���¶��йأ����ʱNH3?H2O�ĵ��볣��Kb=
c(N
| ||
| c(NH3?H2O) |
| ||||
|
| b��10-7 |
| a-b |
�ʴ�Ϊ������
| b��10-7 |
| a-b |

��ϰ��ϵ�д�
 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
�����Ŀ