��Ŀ����
��14�֣���֪����FeS���ܽ���ˮ����ʵ������ȡH2S��SO2�����ԭ���ǣ�
FeS+H2SO4��FeSO4+H2S�� Na2SO3+H2SO4��Na2SO4+H2O+SO2��
������������������������ᷢ����Ӧ��2H2S��SO2��3S��2H2O
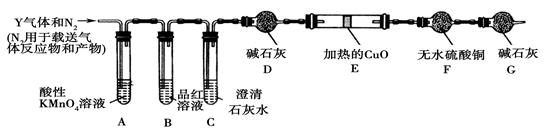
������ͼ��A��D��ʵ��װ�ú��Լ�����ʵ�飬�ش��������⣺
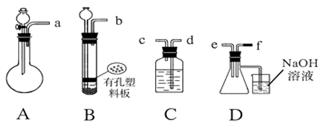
��װ��A����˫�������������ܼ� �� ��װ������
����װһ����ȡSO2�����װ�ã���֤��SO2���������ԡ����л�ԭ�Ժ�Ư����
�ٰ�ʵ��װ������˳��a f�����ܿ�������������ȷ��˳����
f�����ܿ�������������ȷ��˳����
����Cװ���е���ҺΪ ����Ӧ�����Һ��Ϊ��ɫ��˵��SO2���л�ԭ�ԣ�
����Cװ���е���ҺΪ ����Ӧ�����Һ��Ϊ��ɫ��˵��SO2����Ư���ԣ�
�ܵ�D�в��� ����ʱ��˵��SO2���������ԣ�
(3)Dװ���ձ���NaOH��Һ������ ��
FeS+H2SO4��FeSO4+H2S�� Na2SO3+H2SO4��Na2SO4+H2O+SO2��
������������������������ᷢ����Ӧ��2H2S��SO2��3S��2H2O
������ͼ��A��D��ʵ��װ�ú��Լ�����ʵ�飬�ش��������⣺

��װ��A����˫�������������ܼ� �� ��װ������
����װһ����ȡSO2�����װ�ã���֤��SO2���������ԡ����л�ԭ�Ժ�Ư����
�ٰ�ʵ��װ������˳��a
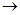 f�����ܿ�������������ȷ��˳����
f�����ܿ�������������ȷ��˳���� | A��befcda | B��adcefb | C��acdfeb | D��acdefb |
����Cװ���е���ҺΪ ����Ӧ�����Һ��Ϊ��ɫ��˵��SO2����Ư���ԣ�
�ܵ�D�в��� ����ʱ��˵��SO2���������ԣ�
(3)Dװ���ձ���NaOH��Һ������ ��
��14�֣�
�� ��Һ©����Բ����ƿ
�� �� AB ������KMnO4��Һ����ˮ����ˮ��
�� Ʒ����Һ ���е���ɫ��������
(3) ����δ��Ӧ��H2S��SO2���壬������Ⱦ����
�� ��Һ©����Բ����ƿ
�� �� AB ������KMnO4��Һ����ˮ����ˮ��
�� Ʒ����Һ ���е���ɫ��������
(3) ����δ��Ӧ��H2S��SO2���壬������Ⱦ����
���⿼���������ʵ������ȡ����Ļ�������������
���ȣ�FeS���ܽ���ˮ����Na2SO3������ˮ��������ȡH2S��װ����B������SO2��װ��ΪA��
�ڶ���Ϊ��֤��SO2�������ԣ�Ӧ����Dװ�õ���ƿ�л�������壻Ϊ��֤���仹ԭ�ԣ�����C�Լ�ƿ��ע������������������KMnO4��Һ����ˮ�ȣ�Ϊ��֤����Ư���ԣ�����Cװ���м���Ʒ����Һ�����顣
������������Dװ���еļ���Һ��β�����յ����á�
���ȣ�FeS���ܽ���ˮ����Na2SO3������ˮ��������ȡH2S��װ����B������SO2��װ��ΪA��
�ڶ���Ϊ��֤��SO2�������ԣ�Ӧ����Dװ�õ���ƿ�л�������壻Ϊ��֤���仹ԭ�ԣ�����C�Լ�ƿ��ע������������������KMnO4��Һ����ˮ�ȣ�Ϊ��֤����Ư���ԣ�����Cװ���м���Ʒ����Һ�����顣
������������Dװ���еļ���Һ��β�����յ����á�

��ϰ��ϵ�д�
 Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
�����Ŀ
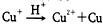 ����NH4CuSO4�м�������3mol/L���������������ȷ����
����NH4CuSO4�м�������3mol/L���������������ȷ����