��Ŀ����
��VL�ܱ������У�ͨ��0.2mol SO2��0.2molSO3���壬��һ�������·�����Ӧ��2SO2��O22SO3��ƽ��ʱSO3Ϊamol������ͬ�¶��°����������VL�ܱ������з�����ʼ���ʣ�ƽ��ʱ�й�SO3����ȷ������
A.����0.2mol SO2��0.1molO2��0.1molSO3���ﵽƽ��ʱSO3��С��amol
B.����0.2mol SO2��0.1molO2��0.2molSO3���ﵽƽ��ʱSO3��С��amol
C.����0.4mol SO2��0.1molO2���ﵽƽ��ʱSO3�����0.4mol
D.����0.2mol SO2��0.1molO2���ﵽƽ��ʱSO3��С��amol
D
����:�ڵ��µ�����Ҫʹƽ���Ч���ͱ���������ʼ���ʵ�����ȡ���ʼ�����൱��0.4mol SO2��0.1molO2��A���൱��0.3mol SO2��0.15molO2���������ȣ��൱��������������Ũ�ȣ���߶��������ת���ʣ����ƽ��ʱSO3��һ��С��amol��B���൱��0.4mol SO2��0.2molO2�����ƽ��ʱSO3�ش���amol����Ϊ��Ӧ�ǿ��淴Ӧ�����ƽ��ʱSO3�����ܵ���0.4mol��D�൱�ڴ�ԭƽ����ϵ�г��0.2mol SO2��ƽ�����淴Ӧ�����ƶ������Դ�D��ȷ��

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д���VL�ܱ������У�ͨ��0.2mol SO2��0.2mol SO3���壬��һ�������·�����Ӧ��2SO2��O2 2SO3��ƽ��ʱSO3Ϊamol������ͬ�¶��°����������VL�ܱ������з�����ʼ���ʣ�ƽ��ʱ�й�SO3����ȷ������
2SO3��ƽ��ʱSO3Ϊamol������ͬ�¶��°����������VL�ܱ������з�����ʼ���ʣ�ƽ��ʱ�й�SO3����ȷ������
| A������0.2mol SO2��0.1molO2��0.1mol SO3���ﵽƽ��ʱSO3��С��amol |
| B������0.2mol SO2��0.1molO2��0.2mol SO3���ﵽƽ��ʱSO3��С��amol |
| C������0.4mol SO2��0.1molO2���ﵽƽ��ʱSO3�����0.4mol |
| D������0.2mol SO2��0.1molO2���ﵽƽ��ʱSO3��С��amol |
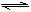 2SO3��ƽ��ʱSO3Ϊamol������ͬ�¶��·ֱ����������VL�ܱ������з�����ʼ���ʣ�ƽ��ʱ�й�SO3�����ʵ���������ȷ���ǣ� ����
2SO3��ƽ��ʱSO3Ϊamol������ͬ�¶��·ֱ����������VL�ܱ������з�����ʼ���ʣ�ƽ��ʱ�й�SO3�����ʵ���������ȷ���ǣ� ����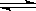 2SO3��ƽ��ʱSO3Ϊamol������ͬ�¶��°����������VL�ܱ������з�����ʼ���ʣ�ƽ��ʱ�й�SO3����ȷ������
2SO3��ƽ��ʱSO3Ϊamol������ͬ�¶��°����������VL�ܱ������з�����ʼ���ʣ�ƽ��ʱ�й�SO3����ȷ������