��Ŀ����
���ͷ��ͨ����������ء��������̡�������ʡ�ij�о���ѧϰС����л��ͷ���й����ʵ�ʵ��̽����?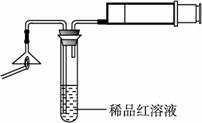
(1)������ͷ�к�����?
����������������ͼ��ʾʵ��װ���е�©�����棬��һ��ȼ�ŵĻ���ȼ����������ע���������������û��ȼ�ղ���������ͨ��ϡƷ����Һ���۲쵽Ʒ����Һ��ɫ��?
��ȼ�ղ�����������һ������������������?
�ڿ���������Թ���Ʒ����Һ���Լ�������������(����)��?
A.ϡ�������������Һ? B.����ʯ��ˮ? C.ϡ��ˮ? D.�ռ���Һ?
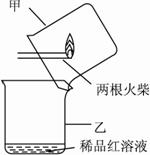
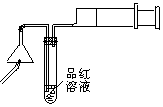
��ijͬѧ���������ͼ��ʾ��������ʵ���Ϊ��㡣���IJ����ǣ�?
i.��ͼ����ʾ���ͷȼ���꣬�����Ƴ����?
ii.����������������������������������?
(2)�ⶨ���ͷ��KClO3�ĺ���?
��Ҫʵ�鲽�����£�?
��.��ȡ���ͷ��С�����飬�Ƶ�����Ϊ2.45 g��?
��.����������ˮ��ֽ��ݺ���ˡ�ϴ�Ӳ�����?
��.��װ����Һ��ϴ��Һ���ձ��м��������NaNO2��Һ��AgNO3��Һ��ϡ���ᣬ���裬��ַ�Ӧ���ˡ�ϴ�ӳ�����?
��.���������Ƶ�������Ϊ1.435 g��?
��ʵ���з����ķ�Ӧ�ǣ�KClO3+3NaNO2+AgNO3��AgCl��+3NaNO3+KNO3,����NaNO2
����������������������Ӧ��AgNO3��NaNO2����Ҫ������ԭ��������������������������
��ʵ���û��ͷ��KClO3����������Ϊ����������?
������ڢ�����δϴ�Ӳ��������KClO3������������������������(�ƫ��ƫС������Ӱ�족����ͬ)������ڢ����У�δϴ��AgCl���������KClO3������������������������������
(1)��SO2(���������)?
��A��C?
��Ѹ�ٽ����ձ��������ձ��ϣ���������ձ�(����������Ҳ�ɸ���)?
(2)�ٻ�ԭ?
ȷ��KClO3����Ԫ��ȫ��ת��ΪAgCl����?
��50%?
��ƫСƫ��
������(1)����ɿ�KClO3Ϊ��������MnO2Ϊ������SΪ��ȼ���ȼ�ղ�����SO2��?
SO2���˿���Ʒ������⣬������SO2ˮ��Һ��ԭ�Խ�ǿ��������ɫ�����������棬�ʿ�ѡϡKMnO4��Һ��ϡ��ˮ��?
����ȼ�ղ���SO2�ܶȱȿ����ʿ�Ѹ�ٽ����ձ��������ձ��ϣ�����SO2�ܶ�ʹSO2�Ӵ�Ʒ�죬�������?
(2)��NaNO2��Ӧ���ΪNaNO3��NԪ�ػ��ϼ����ߣ�Ϊ��ԭ�������߹���ʹKClO3��ַ�Ӧ��
���ھ�Clԭ���غ�,1.435 gΪAgCl��n(KClO3)= n(AgCl)=0.001 mol��m(KClO3)=1.225 g��
�۲�ϴ��������KClO3�в�����MnO2�����������յ�m(KClO3)��С��?
��ϴAgCl�����������ʸ��ţ�ʹm(AgCl)��������m(KClO3)����

 һ����������ϵ�д�
һ����������ϵ�д�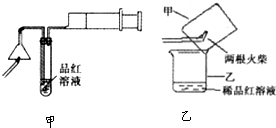

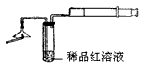
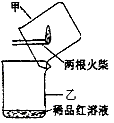
 �ż�����ͷ�к�����
�ż�����ͷ�к�����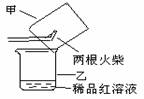 A��ϡ�������������Һ B������ʯ��ˮ C��ϡ��ˮ D���ռ���Һ
A��ϡ�������������Һ B������ʯ��ˮ C��ϡ��ˮ D���ռ���Һ