��Ŀ����
ijС��ͬѧ���о�SO2�����ʡ�
��1������صĺ������ʷ�Ϊ���±���ʾ��3�飬��2��������X�Ļ�ѧʽ������������
|
��1�� |
��2�� |
��3�� |
|
S�����ʣ� |
SO2��X��Na2SO3��NaHSO3 |
SO3��H2SO4��Na2SO4��NaHSO4 |
��2��������ͼ��ʾ��װ���о�SO2�����ʣ�
���۵㣺SO2��76.1�棬SO3 16.8�棻�е㣺SO2��10�棬SO3 45�棩
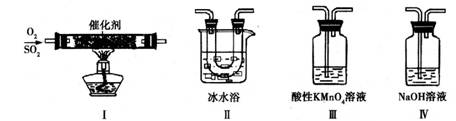
��װ�â�ģ�ҵ������SO2�������ķ�Ӧ���仯ѧ����ʽ��������������
�ڼ�ͬѧ������˳������װ�ã�װ�â������������������������
װ�â�����Һ����ɫ������Mn2+��ͬʱpH���ͣ���÷�Ӧ�����ӷ���ʽ��
����������������������������
����ͬѧ������˳������װ�ã���װ�â�����70mL 2mol��L��1NaOH��Һ����Ӧ������5.12g����װ�â��з�����Ӧ�Ļ�ѧ����ʽ������������������
���𰸡�
��8�֣�
��1��H2SO3��1�֣�
��2����2SO2+O2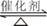 2SO3��1�֣�
2SO3��1�֣�
������SO3����SO3��SO2��O2���루2�֣�
5SO2+2MnO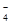 +2H2O
+2H2O 5SO
5SO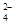 +2Mn2++4H+��2�֣�
+2Mn2++4H+��2�֣�
��4SO2+7NaOH 3Na2SO3+NaHSO3+3H2O��2�֣�
3Na2SO3+NaHSO3+3H2O��2�֣�
��������

��ϰ��ϵ�д�
�����Ŀ
 �����ܺ���CO
�����ܺ���CO
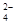 �����ӷ���ʽΪ_____________________________________��
�����ӷ���ʽΪ_____________________________________��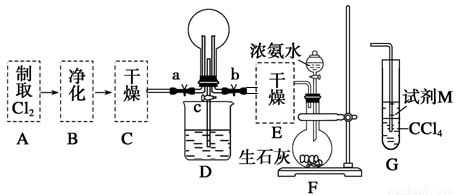
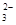 �����ܺ���CO
�����ܺ���CO
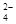 �����ӷ���ʽΪ_____________________________________��
�����ӷ���ʽΪ_____________________________________��