��Ŀ����
ij�о���ѧϰС����ʵ���Һϳ���һ������A��
��1����������A����Է�������������100��A��C��H�����������ֱ�Ϊ��
w(C)��69.76%��w(H)��11.63%������ȫȼ�պ����ֻ��CO2��H2O��
��A��Ħ������Ϊ __________��
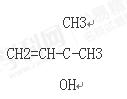
��2��A�ĺ˴Ź�����������ͼ��ʾ����A���Ժͽ����Ʒ�Ӧ����H2����������Cu�������±�������������ʾ���ǻ���̼̼˫�������Ľṹ���ȶ���

�����������Ϣд��A�Ľṹ��ʽ ��
��3��A��ij��ͬ���칹��B�����в���֧�����ܷ���������Ӧ��
��д��B����������Ӧ�Ļ�ѧ����ʽ
��1����������A����Է�������������100��A��C��H�����������ֱ�Ϊ��
w(C)��69.76%��w(H)��11.63%������ȫȼ�պ����ֻ��CO2��H2O��
��A��Ħ������Ϊ __________��
��2��A�ĺ˴Ź�����������ͼ��ʾ����A���Ժͽ����Ʒ�Ӧ����H2����������Cu�������±�������������ʾ���ǻ���̼̼˫�������Ľṹ���ȶ���

�����������Ϣд��A�Ľṹ��ʽ ��
��3��A��ij��ͬ���칹��B�����в���֧�����ܷ���������Ӧ��
��д��B����������Ӧ�Ļ�ѧ����ʽ
��1��86g/mol
CH3
�� CH2=CH-C-CH3
OH
��3�� CH3CH2CH2CH2CHO+ 2Ag(NH3)2OH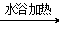 CH3CH2CH2CH2COONH4+2Ag+3NH3+H2O
CH3CH2CH2CH2COONH4+2Ag+3NH3+H2O
CH3
�� CH2=CH-C-CH3
OH
��3�� CH3CH2CH2CH2CHO+ 2Ag(NH3)2OH
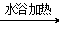 CH3CH2CH2CH2COONH4+2Ag+3NH3+H2O
CH3CH2CH2CH2COONH4+2Ag+3NH3+H2O�����������A����Է�������������100��A��C��H�����������ֱ�Ϊ��w(C)��69.76%��w(H)��11.63%��֪������������Ϊ1-69.76%-11.63%=18.61%������Է�������С��100�����̼�ĸ���Ϊ5������Ϊ1�����Ϊ10����A�ķ���ʽΪC5H10O�ʣ�����Ħ������Ϊ86g/mol����A���Ժͽ����Ʒ�Ӧ����H2����������Cu�������±���������֪A��Ӧ�����ǻ����������ǻ�������̼��û����ԭ�ӡ���֪A�ĽṹΪ
 ����A��ij��ͬ���칹��B�����в���֧�����ܷ���������Ӧ��֪B�ĽṹΪCH3CH2CH2CH2CHO
����A��ij��ͬ���칹��B�����в���֧�����ܷ���������Ӧ��֪B�ĽṹΪCH3CH2CH2CH2CHO
��ϰ��ϵ�д�
 ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д�
�����Ŀ
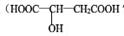 ��������ˮ���ǹ������Ӽ�
��������ˮ���ǹ������Ӽ�