��Ŀ����
�������ӷ���ʽ��д��ȷ����
4Fe2����2Br����3Cl2��4Fe3����6Cl����Br2
| A����Pt�缫���������MgC12��Һ��2H2O��2Cl��ͨ��H2����C12����2OH�� |
| B�������ʵ�����Ba(OH)2��(NH4)2Fe(SO4)2����Һ�з�Ӧ�� Ba2����2OH����2NH4����SO42����BaSO4����2 NH3��H2O |
| C����Na2O2����Ͷ��H218O��2H218O+2 Na2O2��4OH����4Na����18O2�� |
| D������0.4 mol FeBr2����Һ��ͨ��0.3 mol Cl2��ַ�Ӧ�� |
D
A��Ӧ������������þ����������ȷ��A�����������Dz���ģ�Ӧ���������ᱵ����������������������ȷ���������ƺ�ˮ��Ӧ�У��������Ƽ��������������ǻ�ԭ�������ˮ�е���ԭ�ӽ������������У�����ȷ��������ȷ�Ĵ�ѡD��

��ϰ��ϵ�д�
�����Ŀ
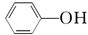 ��CO��
��CO�� 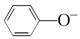 ��HCO
��HCO H2CO3+ 2OH��
H2CO3+ 2OH��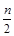 ʱ����Ӧ�����ӷ���ʽΪ�� ��
ʱ����Ӧ�����ӷ���ʽΪ�� �� BaSO4�� + NH3�� + H2O
BaSO4�� + NH3�� + H2O