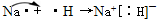��Ŀ����
��ͼ��Ԫ�����ڱ���һ����
��1������Ԫ���У�����s��Ԫ�ص��� ����Ԫ�ط��ţ�
��2��Ԫ�آ���̬�⻯�������ԭ���� �Թ¶Ե��ӣ���VSEPRģ��Ϊ ��������ͭ��Һ����μ�����ˮ��Һ���ɹ۲쵽������Ϊ ��
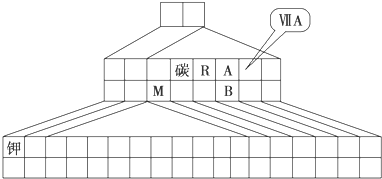
��3��д��Ԫ�آ�Ļ�̬ԭ�ӵļ۵����Ų�ʽ ��ָ���������ڱ��е�λ�� �����Ӹֹ�ʱ�������â��ijЩ��������ߵĵ����ڸ����·�����Ӧ����д������һ�ַ�Ӧ�Ļ�ѧ����ʽ ��
��4���٢ۢ�����Ԫ�ؿ����γɶ����л���������ӣ��������ԭ�������ٵ�һ��������װ��ʱ�γɵ���Ҫ������Ⱦ���д�����ĵ���ʽ ��
��5��Ԫ�آٿ�����Ԫ�آ��γɻ������д�����ĵ���ʽ ��
��6��ijЩ��ͬ��Ԫ�ص�����Ҳ��һ���������ԣ����ϱ���Ԫ�آ���Ԫ�آڵ��������������Ƶ����ʣ���д��Ԫ�آڵ�����������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ ��

��1������Ԫ���У�����s��Ԫ�ص���
��2��Ԫ�آ���̬�⻯�������ԭ����
��3��д��Ԫ�آ�Ļ�̬ԭ�ӵļ۵����Ų�ʽ
��4���٢ۢ�����Ԫ�ؿ����γɶ����л���������ӣ��������ԭ�������ٵ�һ��������װ��ʱ�γɵ���Ҫ������Ⱦ���д�����ĵ���ʽ
��5��Ԫ�آٿ�����Ԫ�آ��γɻ������д�����ĵ���ʽ
��6��ijЩ��ͬ��Ԫ�ص�����Ҳ��һ���������ԣ����ϱ���Ԫ�آ���Ԫ�آڵ��������������Ƶ����ʣ���д��Ԫ�آڵ�����������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ
��������Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪBe����ΪC����ΪN����ΪO����ΪNa����ΪAl����ΪCl����ΪTi����ΪFe��
��1��������ĵ���Ϊs���ӣ�Ϊs��Ԫ�أ�
��2��Ԫ�آ���̬�⻯��ΪNH3����1�Թ¶Ե��ӣ���3�����۵�����Nԭ��Ϊsp3�ӻ�������ͭ�мӰ�ˮ�������ɳ��������������ӣ�
��3��Fe��ԭ������Ϊ26���۵���Ϊ3d64s2��λ�ڵ������ڵڢ����壬���ijЩ��������ߵĵ����ڸ����·������ȷ�Ӧ��
��4�����ԭ�������ٵ�һ��������װ��ʱ�γɵ���Ҫ������Ⱦ��ΪHCHO��
��5��Ԫ�آٿ�����Ԫ�آ��γɻ�����ΪNaH��Ϊ���ӻ����
��6��Ԫ�آ���Ԫ�آڵ��������������Ƶ����ʣ�Ԫ�آڵ�����������NaOH��Һ��Ӧ����Na2BeO2��ˮ��
��1��������ĵ���Ϊs���ӣ�Ϊs��Ԫ�أ�
��2��Ԫ�آ���̬�⻯��ΪNH3����1�Թ¶Ե��ӣ���3�����۵�����Nԭ��Ϊsp3�ӻ�������ͭ�мӰ�ˮ�������ɳ��������������ӣ�
��3��Fe��ԭ������Ϊ26���۵���Ϊ3d64s2��λ�ڵ������ڵڢ����壬���ijЩ��������ߵĵ����ڸ����·������ȷ�Ӧ��
��4�����ԭ�������ٵ�һ��������װ��ʱ�γɵ���Ҫ������Ⱦ��ΪHCHO��
��5��Ԫ�آٿ�����Ԫ�آ��γɻ�����ΪNaH��Ϊ���ӻ����
��6��Ԫ�آ���Ԫ�آڵ��������������Ƶ����ʣ�Ԫ�آڵ�����������NaOH��Һ��Ӧ����Na2BeO2��ˮ��
����⣺��Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪBe����ΪC����ΪN����ΪO����ΪNa����ΪAl����ΪCl����ΪTi����ΪFe��
��1��������ĵ���Ϊs���ӣ�Ϊs��Ԫ�أ���٢ڢ��ϣ���H��Be��NaΪs��Ԫ�أ��ʴ�Ϊ��H��Be��Na��
��2��Ԫ�آ���̬�⻯��ΪNH3����1�Թ¶Ե��ӣ���3�����۵�����Nԭ��Ϊsp3�ӻ���VSEPRģ��Ϊ�������ͣ�����ͭ�мӰ�ˮ��������������ͭ���������������ӣ��ɹ۲쵽��������ɫ����������ܽ�õ���ɫ��Һ���ʴ�Ϊ��1�������壻��������ɫ����������ܽ�õ���ɫ��Һ��
��3��Fe��ԭ������Ϊ26���۵���Ϊ3d64s2��λ�ڵ������ڵڢ����壬���ijЩ��������ߵĵ����ڸ����·������ȷ�Ӧ����2Al+Fe2O3
Al2O3+2Fe��
�ʴ�Ϊ��3d64s2���������ڵڢ����壻2Al+Fe2O3
Al2O3+2Fe��
��4�����ԭ�������ٵ�һ��������װ��ʱ�γɵ���Ҫ������Ⱦ��ΪHCHO�������ʽΪ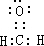 ���ʴ�Ϊ��
���ʴ�Ϊ��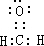 ��
��
��5��Ԫ�آٿ�����Ԫ�آ��γɻ�����ΪNaH��Ϊ���ӻ���������ʽΪNa+[��H]-���ʴ�Ϊ��Na+[��H]-��
��6��Ԫ�آ���Ԫ�آڵ��������������Ƶ����ʣ�Ԫ�آڵ�����������NaOH��Һ��Ӧ����Na2BeO2��ˮ���÷�ӦΪBe��OH��2+2NaOH=Na2BeO2+2H2O��
�ʴ�Ϊ��Be��OH��2+2NaOH=Na2BeO2+2H2O��
��1��������ĵ���Ϊs���ӣ�Ϊs��Ԫ�أ���٢ڢ��ϣ���H��Be��NaΪs��Ԫ�أ��ʴ�Ϊ��H��Be��Na��
��2��Ԫ�آ���̬�⻯��ΪNH3����1�Թ¶Ե��ӣ���3�����۵�����Nԭ��Ϊsp3�ӻ���VSEPRģ��Ϊ�������ͣ�����ͭ�мӰ�ˮ��������������ͭ���������������ӣ��ɹ۲쵽��������ɫ����������ܽ�õ���ɫ��Һ���ʴ�Ϊ��1�������壻��������ɫ����������ܽ�õ���ɫ��Һ��
��3��Fe��ԭ������Ϊ26���۵���Ϊ3d64s2��λ�ڵ������ڵڢ����壬���ijЩ��������ߵĵ����ڸ����·������ȷ�Ӧ����2Al+Fe2O3
| ||
�ʴ�Ϊ��3d64s2���������ڵڢ����壻2Al+Fe2O3
| ||
��4�����ԭ�������ٵ�һ��������װ��ʱ�γɵ���Ҫ������Ⱦ��ΪHCHO�������ʽΪ
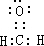 ���ʴ�Ϊ��
���ʴ�Ϊ��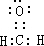 ��
����5��Ԫ�آٿ�����Ԫ�آ��γɻ�����ΪNaH��Ϊ���ӻ���������ʽΪNa+[��H]-���ʴ�Ϊ��Na+[��H]-��
��6��Ԫ�آ���Ԫ�آڵ��������������Ƶ����ʣ�Ԫ�آڵ�����������NaOH��Һ��Ӧ����Na2BeO2��ˮ���÷�ӦΪBe��OH��2+2NaOH=Na2BeO2+2H2O��
�ʴ�Ϊ��Be��OH��2+2NaOH=Na2BeO2+2H2O��
���������⿼��Ԫ�����ڱ���Ӧ�ã�����Ԫ�������ڱ��е�λ�á�ԭ�ӽṹ�����ʡ�Ԫ�ػ�����֪ʶΪ���Ĺؼ�������Ԫ���ƶϼ���ѧ����Ŀ��飬ע�⣨6�������ʵ����ƣ���Ŀ�ѶȲ���

��ϰ��ϵ�д�
 Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�
�����Ŀ
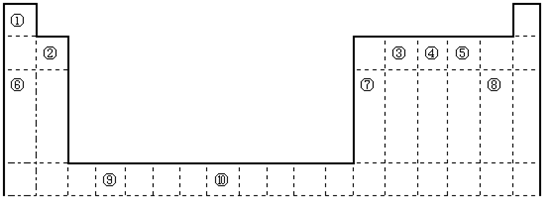
 ��ͼ��Ԫ�����ڱ���һ���֣�X��Y��Z��W��Ϊ������Ԫ�أ�X��Z��������֮��Ϊ21������˵����ȷ���ǣ�������
��ͼ��Ԫ�����ڱ���һ���֣�X��Y��Z��W��Ϊ������Ԫ�أ�X��Z��������֮��Ϊ21������˵����ȷ���ǣ�������| A��XԪ�������γ����������� | B��YԪ�ص�����������ˮ�����Ǻ�������������ǿ�� | C��XԪ�صķǽ����Ա�YԪ�طǽ�����ǿ | D��Z��X���Թ��ۼ�����γ�һ�����ǽ������� |