��Ŀ����
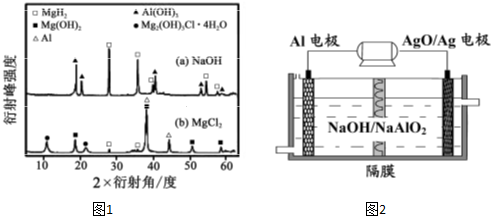
��1�����ǵؿ��к������Ľ����������ᣬȼ��ʱ�������ߣ��ڳ��³�ѹ�£�9g��������ȫȼ�����ɹ�̬��A2O3ʱ�ų�235kJ���ȣ��������Ӧ���Ȼ�ѧ����ʽΪ ����2���й������������ĵ�ұ������֮һ����ұ����ԭ�������������������½��е�⣬��д����ⷨұ�����ķ���ʽΪ ��
��3������;�ܹ㣬�ڵ绯ѧ�г����ڳ䵱�缫���ϣ�������þ�õ������Ӻ������뵽ϡ�����У��������� ����������������Һ�У������ĵ缫��Ӧʽ��
��4���й��״��ĺ�������ƣ��������������ͺ�ˮΪԭ������Ƶ�ԭ��أ����ѵ�����ں�ˮ��ؾ��нϴ�ĵ�ѹ���˵�ص�������ӦʽΪ ��
��5�����ö��Ե缫��NO3-��SO42-��Cl-��Cu2+��Mg2+��H+��ѡ���ʵ���������ɵ���ʣ���������Һ���е��
���������ֱ�ų�H2��O2�������ʵĻ�ѧʽΪ ��
�����������������������ų�O2�������ʵĻ�ѧʽΪ ��
���𰸡���������1��9g�������ʵ���=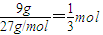 ��9g��������ȫȼ�����ɹ�̬��A2O3ʱ�ų�235kJ���ȣ���4mol����ȫȼ�շų�2820KJ�����������Ȼ�ѧ��Ӧ����ʽ����д����д�����ɣ�
��9g��������ȫȼ�����ɹ�̬��A2O3ʱ�ų�235kJ���ȣ���4mol����ȫȼ�շų�2820KJ�����������Ȼ�ѧ��Ӧ����ʽ����д����д�����ɣ�
��2���������̬��������������������
��3������þ��ϡ���ṹ�ɵ�ԭ����У�þ�����������������������������ӵõ�����������������þ������������Һ���ɵ�ԭ����У�����������þ����������������ʧ���Ӻ����������ӷ�Ӧ����ƫ��������Ӻ�ˮ��
��4�����������ͺ�ˮ���ɵ�ԭ����У���������ʧ���ӷ���������Ӧ��
��5�����ݵ������Һ�����ӵķŵ�˳��������������ȷ����Һ�к��е��������ӣ��Ӷ�ȷ�������ʵĻ�ѧʽ��
����⣺��1��9g�������ʵ���=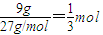 ��9g��������ȫȼ�����ɹ�̬��A2O3ʱ�ų�235kJ���ȣ���4mol����ȫȼ�շų�2820KJ�������������Ȼ�ѧ��Ӧ����ʽΪ��
��9g��������ȫȼ�����ɹ�̬��A2O3ʱ�ų�235kJ���ȣ���4mol����ȫȼ�շų�2820KJ�������������Ȼ�ѧ��Ӧ����ʽΪ��
4Al��s��+3O2��g��=2Al2O3��s������H=2820kJ/mol��
�ʴ�Ϊ��4Al��s��+3O2��g��=2Al2O3��s������H=2820kJ/mol��
��2���������̬����������������������ⷽ��ʽΪ2Al2O3 4Al+3O2����
4Al+3O2����
�ʴ�Ϊ��2Al2O3 4Al+3O2����
4Al+3O2����
��3������þ��ϡ���ṹ�ɵ�ԭ����У������������ӵõ��������������缫��ӦʽΪ2H++2e-=H2��������þ������������Һ���ɵ�ԭ����У�����������þ����������������ʧ���Ӻ����������ӷ�Ӧ����ƫ��������Ӻ�ˮ��
�缫��ӦʽΪAl-3e-+4HO-=AlO2-+2H2O��
�ʴ�Ϊ��2H++2e-=H2����Al-3e-+4HO-=AlO2-+2H2O��
��4�����������ͺ�ˮ���ɵ�ԭ����У���������ʧ�������������ӣ��缫��ӦʽΪAl-3e-=Al3+��
�ʴ�Ϊ��Al-3e-=Al3+��
��5�����������ֱ�ų�H2��O2���������������ӷŵ磬���������������ӷŵ磬�����������ӷŵ�˳��֪����Һ�д��ڵ�������Ϊ����������ӣ�������Ϊ�����ӻ���ý������ӣ����Ե���ʵĻ�ѧʽΪHNO3��H2SO4��Mg��NO3��2��MgSO4��
�ʴ�Ϊ��HNO3��H2SO4��Mg��NO3��2��MgSO4��
�������������������������ӵķŵ�˳��֪����Һ�к���H֮��IJ����ý��������ӣ������ų�O2������Һ�к��к���������ӣ����Ե���ʵĻ�ѧʽΪCu��NO3��2��CuSO4��
�ʴ�Ϊ��Cu��NO3��2��CuSO4��
���������⿼�����Ȼ�ѧ��Ӧ����ʽ����д���缫��Ӧʽ����д������ʵ�ȷ����֪ʶ�㣬�״���������þ������������Һ���ɵ�ԭ�������������ȷ����һ����˵�ϻ��ý���������������ԭ����������������Ʒ���������ԭ��Ӧ����þ����Ӧ����������������þ��������
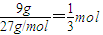 ��9g��������ȫȼ�����ɹ�̬��A2O3ʱ�ų�235kJ���ȣ���4mol����ȫȼ�շų�2820KJ�����������Ȼ�ѧ��Ӧ����ʽ����д����д�����ɣ�
��9g��������ȫȼ�����ɹ�̬��A2O3ʱ�ų�235kJ���ȣ���4mol����ȫȼ�շų�2820KJ�����������Ȼ�ѧ��Ӧ����ʽ����д����д�����ɣ���2���������̬��������������������
��3������þ��ϡ���ṹ�ɵ�ԭ����У�þ�����������������������������ӵõ�����������������þ������������Һ���ɵ�ԭ����У�����������þ����������������ʧ���Ӻ����������ӷ�Ӧ����ƫ��������Ӻ�ˮ��
��4�����������ͺ�ˮ���ɵ�ԭ����У���������ʧ���ӷ���������Ӧ��
��5�����ݵ������Һ�����ӵķŵ�˳��������������ȷ����Һ�к��е��������ӣ��Ӷ�ȷ�������ʵĻ�ѧʽ��
����⣺��1��9g�������ʵ���=
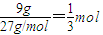 ��9g��������ȫȼ�����ɹ�̬��A2O3ʱ�ų�235kJ���ȣ���4mol����ȫȼ�շų�2820KJ�������������Ȼ�ѧ��Ӧ����ʽΪ��
��9g��������ȫȼ�����ɹ�̬��A2O3ʱ�ų�235kJ���ȣ���4mol����ȫȼ�շų�2820KJ�������������Ȼ�ѧ��Ӧ����ʽΪ��4Al��s��+3O2��g��=2Al2O3��s������H=2820kJ/mol��
�ʴ�Ϊ��4Al��s��+3O2��g��=2Al2O3��s������H=2820kJ/mol��
��2���������̬����������������������ⷽ��ʽΪ2Al2O3
 4Al+3O2����
4Al+3O2�����ʴ�Ϊ��2Al2O3
 4Al+3O2����
4Al+3O2������3������þ��ϡ���ṹ�ɵ�ԭ����У������������ӵõ��������������缫��ӦʽΪ2H++2e-=H2��������þ������������Һ���ɵ�ԭ����У�����������þ����������������ʧ���Ӻ����������ӷ�Ӧ����ƫ��������Ӻ�ˮ��
�缫��ӦʽΪAl-3e-+4HO-=AlO2-+2H2O��
�ʴ�Ϊ��2H++2e-=H2����Al-3e-+4HO-=AlO2-+2H2O��
��4�����������ͺ�ˮ���ɵ�ԭ����У���������ʧ�������������ӣ��缫��ӦʽΪAl-3e-=Al3+��
�ʴ�Ϊ��Al-3e-=Al3+��
��5�����������ֱ�ų�H2��O2���������������ӷŵ磬���������������ӷŵ磬�����������ӷŵ�˳��֪����Һ�д��ڵ�������Ϊ����������ӣ�������Ϊ�����ӻ���ý������ӣ����Ե���ʵĻ�ѧʽΪHNO3��H2SO4��Mg��NO3��2��MgSO4��
�ʴ�Ϊ��HNO3��H2SO4��Mg��NO3��2��MgSO4��
�������������������������ӵķŵ�˳��֪����Һ�к���H֮��IJ����ý��������ӣ������ų�O2������Һ�к��к���������ӣ����Ե���ʵĻ�ѧʽΪCu��NO3��2��CuSO4��
�ʴ�Ϊ��Cu��NO3��2��CuSO4��
���������⿼�����Ȼ�ѧ��Ӧ����ʽ����д���缫��Ӧʽ����д������ʵ�ȷ����֪ʶ�㣬�״���������þ������������Һ���ɵ�ԭ�������������ȷ����һ����˵�ϻ��ý���������������ԭ����������������Ʒ���������ԭ��Ӧ����þ����Ӧ����������������þ��������

��ϰ��ϵ�д�
�����Ŀ