��Ŀ����
��l6�֣�ijУ��ѧ�о���ѧϰС���ͬѧ��ѧϰ�˰�������ʱ���ۣ���Ȼ�������л�ԭ�ԣ��ܷ���H2������ԭCuO�أ��������ʵ����ȡ������̽���������⡣��������С��Ļ����������о���
��1����ȡ����
��д��ʵ����ȡ�����Ļ�ѧ����ʽ ��
����ʵ�����У���������Ũ��ˮ�� ����дһ���Լ���������ȡ����������
����ͬѧģ���ű���ʳ��ˮ�ռ������ķ����������ű����Ȼ�����Һ�ķ����ռ�����������Ϊ���ܷ�ﵽĿ�ģ� ����ܡ����������� ��
��2����С����ijͬѧ�������ͼ��ʾ��ʵ��װ�ã��гּ�β������װ��δ��������̽�������Ļ�ԭ�ԣ�
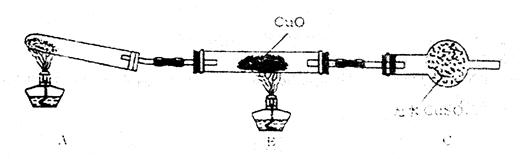
��װ�����������һ��ȱ�ݣ�Ϊ��֤ʵ������ȷ�ԣ��Ը�װ�õĸĽ���ʩ��
��
�����øĽ����װ�ý���ʵ�飬�۲쵽CuO��Ϊ��ɫ���ʣ���ˮCuSO4����ɫ��ͬʱ����һ������Ⱦ������д��������CuO��Ӧ�Ļ�ѧ����ʽ ��
��3����������
��ͬѧ��Ϊ��NH3��CuO��Ӧ���ɵĺ�ɫ�����п��ܺ���Cu2O����֪��Cu2O��һ�ּ����������������Һ�У�Cu+ ���ȶ��Ա�Cu2+ �2Cu+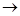 Cu+ Cu2+ �����������һ����ʵ�����ú�ɫ�������Ƿ���Cu2O ��
Cu+ Cu2+ �����������һ����ʵ�����ú�ɫ�������Ƿ���Cu2O ��
��1����ȡ����
��д��ʵ����ȡ�����Ļ�ѧ����ʽ ��
����ʵ�����У���������Ũ��ˮ�� ����дһ���Լ���������ȡ����������
����ͬѧģ���ű���ʳ��ˮ�ռ������ķ����������ű����Ȼ�����Һ�ķ����ռ�����������Ϊ���ܷ�ﵽĿ�ģ� ����ܡ����������� ��
��2����С����ijͬѧ�������ͼ��ʾ��ʵ��װ�ã��гּ�β������װ��δ��������̽�������Ļ�ԭ�ԣ�

��װ�����������һ��ȱ�ݣ�Ϊ��֤ʵ������ȷ�ԣ��Ը�װ�õĸĽ���ʩ��
��
�����øĽ����װ�ý���ʵ�飬�۲쵽CuO��Ϊ��ɫ���ʣ���ˮCuSO4����ɫ��ͬʱ����һ������Ⱦ������д��������CuO��Ӧ�Ļ�ѧ����ʽ ��
��3����������
��ͬѧ��Ϊ��NH3��CuO��Ӧ���ɵĺ�ɫ�����п��ܺ���Cu2O����֪��Cu2O��һ�ּ����������������Һ�У�Cu+ ���ȶ��Ա�Cu2+ �2Cu+
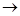 Cu+ Cu2+ �����������һ����ʵ�����ú�ɫ�������Ƿ���Cu2O ��
Cu+ Cu2+ �����������һ����ʵ�����ú�ɫ�������Ƿ���Cu2O ����16�֣�ÿ�� 2�֣�
��1����2NH4Cl+Ca��OH��2 CaCl2+2NH3
CaCl2+2NH3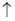 +2H2O
+2H2O
��NaOH�������ʯ�һ��ʯ�ҵȣ�ֻҪ�������֣�����NaOH��Һ����ʯ�ҵȲ����֣�
�۷�����������ˮ���Ȼ��¶�����ˮ�е��ܽ�Ӱ�첻��
��2������װ��A��B֮������װ�м�ʯ�ҵĸ���ܣ�Cװ��֮������β������װ�á�
��3CuO+2NH3 3Cu+N2+3H2O
3Cu+N2+3H2O
��3��ȡ������Ʒ������ϡH2SO4����ϡ���ᣩ������Һ������ɫ��˵����ɫ�����к���CU2O����֮��û��
��1����2NH4Cl+Ca��OH��2
 CaCl2+2NH3
CaCl2+2NH3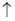 +2H2O
+2H2O ��NaOH�������ʯ�һ��ʯ�ҵȣ�ֻҪ�������֣�����NaOH��Һ����ʯ�ҵȲ����֣�
�۷�����������ˮ���Ȼ��¶�����ˮ�е��ܽ�Ӱ�첻��
��2������װ��A��B֮������װ�м�ʯ�ҵĸ���ܣ�Cװ��֮������β������װ�á�
��3CuO+2NH3
 3Cu+N2+3H2O
3Cu+N2+3H2O��3��ȡ������Ʒ������ϡH2SO4����ϡ���ᣩ������Һ������ɫ��˵����ɫ�����к���CU2O����֮��û��
��1��ʵ���ҳ�����ʯ�Һ��Ȼ�炙�ϼ�����ȡ������ʽΪ��2NH4Cl+Ca��OH��2 CaCl2+2NH3
CaCl2+2NH3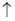 +2H2O����Ϊ��ˮ����������ʴ��ڵ���ƽ��NH3��H2O
+2H2O����Ϊ��ˮ����������ʴ��ڵ���ƽ��NH3��H2O NH4����OH������˿��������������ƻ���ʯ������ȡ����������������������ˮ���Ȼ�臨����ܽ��Ӱ�첻�����ñ����Ȼ�����Һ�ķ����ռ�������
NH4����OH������˿��������������ƻ���ʯ������ȡ����������������������ˮ���Ȼ�臨����ܽ��Ӱ�첻�����ñ����Ȼ�����Һ�ķ����ռ�������
��2������������ͭ��ӦӦ�Ǹ���İ���������Ҫ��β������װ�á�CuO��Ϊ��ɫ���ʣ�˵������ԭ�����˵���ͭ����ˮCuSO4����ɫ��˵����ˮ���ɡ�ͬʱ����һ������Ⱦ�����壬˵���������������ɵ���������ʽΪ2NH3��3CuO N2��3Cu��3H2O��
N2��3Cu��3H2O��
��3����������Һ�У�Cu+ ���ȶ��Ա�Cu2+ �����2Cu+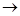 Cu+ Cu2+ ������˿��������ɵ�Cu2+����Һ������ɫ����֤�������õ���һ���������������ᡣ
Cu+ Cu2+ ������˿��������ɵ�Cu2+����Һ������ɫ����֤�������õ���һ���������������ᡣ
 CaCl2+2NH3
CaCl2+2NH3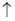 +2H2O����Ϊ��ˮ����������ʴ��ڵ���ƽ��NH3��H2O
+2H2O����Ϊ��ˮ����������ʴ��ڵ���ƽ��NH3��H2O NH4����OH������˿��������������ƻ���ʯ������ȡ����������������������ˮ���Ȼ�臨����ܽ��Ӱ�첻�����ñ����Ȼ�����Һ�ķ����ռ�������
NH4����OH������˿��������������ƻ���ʯ������ȡ����������������������ˮ���Ȼ�臨����ܽ��Ӱ�첻�����ñ����Ȼ�����Һ�ķ����ռ���������2������������ͭ��ӦӦ�Ǹ���İ���������Ҫ��β������װ�á�CuO��Ϊ��ɫ���ʣ�˵������ԭ�����˵���ͭ����ˮCuSO4����ɫ��˵����ˮ���ɡ�ͬʱ����һ������Ⱦ�����壬˵���������������ɵ���������ʽΪ2NH3��3CuO
 N2��3Cu��3H2O��
N2��3Cu��3H2O����3����������Һ�У�Cu+ ���ȶ��Ա�Cu2+ �����2Cu+
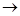 Cu+ Cu2+ ������˿��������ɵ�Cu2+����Һ������ɫ����֤�������õ���һ���������������ᡣ
Cu+ Cu2+ ������˿��������ɵ�Cu2+����Һ������ɫ����֤�������õ���һ���������������ᡣ
��ϰ��ϵ�д�
�����Ŀ