��Ŀ����
��������������ѧ�о��IJ�ʢʱ�ڣ���ѧ���о����֣������������ڵ������ӣ��ɽ���1������ת��Ϊ�������������ɻ�(O![]() )��������������һϵ�����ɻ���һ������������������ھ���һ�������Ļ�����ϵͳ(������ø�Ϳ�������)���ܽ�������ת��Ϊ���Խϵ͵����ʣ���������ܵ����������������ǰ�(NH2OH)�����ķ������Լ��������ϵͳ��O
)��������������һϵ�����ɻ���һ������������������ھ���һ�������Ļ�����ϵͳ(������ø�Ϳ�������)���ܽ�������ת��Ϊ���Խϵ͵����ʣ���������ܵ����������������ǰ�(NH2OH)�����ķ������Լ��������ϵͳ��O![]() ������ԭ����O2�����ǰ���Ӧ����NO
������ԭ����O2�����ǰ���Ӧ����NO![]() ��һ�ֹ������NO
��һ�ֹ������NO![]() �ڶ���������ͦ������������£����ɷۺ��ż��Ⱦ�壬Ⱦ���ڲ���530 nm�����������գ���������ֵ��c(NO
�ڶ���������ͦ������������£����ɷۺ��ż��Ⱦ�壬Ⱦ���ڲ���530 nm�����������գ���������ֵ��c(NO![]() )�����ȣ��Ӷ��ɼ������Ʒ�е�O
)�����ȣ��Ӷ��ɼ������Ʒ�е�O![]() ������ijʵ���������Ϸ�������������Һ��c(NO
������ijʵ���������Ϸ�������������Һ��c(NO![]() )��2.500��10��3 mol��L��1��
)��2.500��10��3 mol��L��1��
(1)����ݲⶨԭ��д���йط�Ӧ�����ӷ���ʽ________��
(2)�������Ʒ��ʱc(O![]() )��________��
)��________��
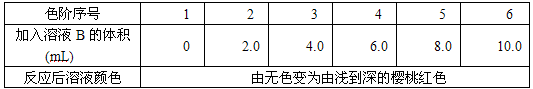
(3)�����ǰ��������ⶨO![]() ʱ���������ɵĹ���������Ϊ������ѡ��������ԭ�����ж�������(��KMnO4����Һ���еζ�)
ʱ���������ɵĹ���������Ϊ������ѡ��������ԭ�����ж�������(��KMnO4����Һ���еζ�)
����д����ⶨԭ���ķ�Ӧ����ʽ________��
�ڲⶨ���õ���Ҫ��������Ϊ________���ζ��յ������Ϊ��________
�𰸣�

��ϰ��ϵ�д�
�����Ŀ
 ��ѧ���о����֣������������ڵ������ӣ��ɽ���1������ת��Ϊ�������������ɻ���O2������������������һϵ�����ɻ���һ������������������ھ���һ�������Ļ�����ϵͳ��������ø�Ϳ������������ܽ�������ת��Ϊ���Խϵ͵����ʣ���������ܵ����������������ǰ���NH2OH�������ķ������Լ��������ϵͳ��O2��������ԭ����O2�����ǰ���Ӧ����NO2����һ�ֹ������NO2���ڶ���������ͦ������������£����ɷۺ��ż��Ⱦ�壬Ⱦ���ڲ���530nm�����������գ���������ֵ��c(NO2��)�����ȣ��Ӷ��ɼ������Ʒ�е�O2��������ijʵ���������Ϸ�������������Һ��c(NO2��)��2.500��10-3 mol?L-1��
��ѧ���о����֣������������ڵ������ӣ��ɽ���1������ת��Ϊ�������������ɻ���O2������������������һϵ�����ɻ���һ������������������ھ���һ�������Ļ�����ϵͳ��������ø�Ϳ������������ܽ�������ת��Ϊ���Խϵ͵����ʣ���������ܵ����������������ǰ���NH2OH�������ķ������Լ��������ϵͳ��O2��������ԭ����O2�����ǰ���Ӧ����NO2����һ�ֹ������NO2���ڶ���������ͦ������������£����ɷۺ��ż��Ⱦ�壬Ⱦ���ڲ���530nm�����������գ���������ֵ��c(NO2��)�����ȣ��Ӷ��ɼ������Ʒ�е�O2��������ijʵ���������Ϸ�������������Һ��c(NO2��)��2.500��10-3 mol?L-1��