��Ŀ����
8��������������ʽ����ƽ������£�N2H4����Ϊȼ�ϣ�NO2��Ϊ����������Ӧ����N2��ˮ��������֪��N2��g��+2O2��g��=2NO2��g������H=+67.7kJ/mol
N2H4��g��+O2��g��=N2��g��+2H2O��g������H=-534kJ/mol
���й����º�NO2��Ӧ���Ȼ�ѧ����ʽ�У���ȷ���ǣ�������
| A�� | 2N2H4��g��+2NO2��g��=3N2��g��+4H2O��l������H=-1135.7 kJ/mol | |
| B�� | N2H4��g��+NO2��g��=3/2N2��g��+2H2O��g������H=-567.85 kJ/mol | |
| C�� | N2H4��g��+NO2��g��=3/2N2��g��+2H2O��l������H=-1135.7 kJ/mol | |
| D�� | 2N2H4��g��+2NO2��g��=3N2��g��+4H2O��g������H=+1135.7 kJ/mol |
���� ���ݸ�˹���ɣ�����֪�Ȼ�ѧ��Ӧ����ʽ�����ʵ���ϵ�����мӼ�����Ŀ���Ȼ�ѧ����ʽ����Ӧ��Ҳ������Ӧ��ϵ��������Ӧ�ļӼ����ݴ���д��
��� �⣺��֪���١�N2��g��+2O2��g��=2NO2��g����H=+67.7kJ•mol-l��
�ڡ�N2H4��g��+O2��g��=N2��g��+2H2O��g����H=-534kJ•mol-l��
�ɸ�˹���ɣ��ڡ�2-�ٵ�2N2H4��g��+2NO2��g��=3N2��g��+4H2O��g�����ʸ÷�Ӧ�ġ�H=2����-534kJ•mol-1��-67.7kJ•mol-1��=-1135.7 kJ•mol-1��
��2N2H4��g��+2NO2��g��=3N2��g��+4H2O��g����H=-1135.7 kJ•mol-1��
��ѡ��A��
���� ���⿼�鷴Ӧ�ȵ��йؼ��㡢�Ȼ�ѧ����ʽ����д���Ѷ��еȣ������˹�����ǽ���Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
18���������е��Ӳ�ṹ��ԭ�ӣ�����ӦԪ��һ������ͬһ������ǣ�������
| A�� | 3p�������2��δ�ɶԵ��ӵ�ԭ�Ӻ�4p�������2��δ�ɶԵ��ӵ�ԭ�� | |
| B�� | 3p�����ֻ��1���չ����ԭ�Ӻ�4p�����ֻ��1���չ����ԭ�� | |
| C�� | ���������Ų�Ϊ1s2��ԭ�Ӻ����������Ų�Ϊ2s22p6��ԭ�� | |
| D�� | ���������Ų�Ϊ1s2��ԭ�Ӻ����������Ų�Ϊ2s2��ԭ�� |
19�� ����ȼ�ϵ���Ƿ�����ɫ��ѧ��������ͷ���װ�ã���ͼΪ���ʾ��ͼ�������й�����ȼ�ϵ�ص�˵����ȷ���ǣ�������
����ȼ�ϵ���Ƿ�����ɫ��ѧ��������ͷ���װ�ã���ͼΪ���ʾ��ͼ�������й�����ȼ�ϵ�ص�˵����ȷ���ǣ�������
 ����ȼ�ϵ���Ƿ�����ɫ��ѧ��������ͷ���װ�ã���ͼΪ���ʾ��ͼ�������й�����ȼ�ϵ�ص�˵����ȷ���ǣ�������
����ȼ�ϵ���Ƿ�����ɫ��ѧ��������ͷ���װ�ã���ͼΪ���ʾ��ͼ�������й�����ȼ�ϵ�ص�˵����ȷ���ǣ�������| A�� | �õ�ع���ʱ����ת���ɻ�ѧ�� | |
| B�� | �õ����a������ | |
| C�� | ���·�еĵ����ɵ缫bͨ����������a | |
| D�� | �õ�ص��ܷ�ӦΪ��2H2+O2�T2H2O |
16����Ӧ3Fe��s��+4H2O��g��$\stackrel{����}{?}$Fe3O4��s��+4H2��g����һ�ɱ��ݻ����ܱ������н��У����������ĸı��ʹ��ѧ��Ӧ���ʼӿ���ǣ�������
| A�� | ����Fe���� | |
| B�� | ���������ݻ���Сһ�� | |
| C�� | �����ݻ����䣬����N2ʹ��ϵѹǿ���� | |
| D�� | ѹǿ���䣬����N2ʹ�����ݻ����� |
3������ˮ��Һ�д��������һ�������ǣ�������
| A�� | K+��H+��NO3-��SiO32- | B�� | H+��NH4+��Al3+��SO42- | ||
| C�� | Fe2+��H+��ClO-��SO42- | D�� | AlO2-��Mg2+��HCO3-��NO3- |
20�����и߷��ӻ���������Ӧ�Ľṹ��Ԫ��ȷ���ǣ�������
| A�� | ������ϩ��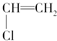 | B�� | �۱���ϩ��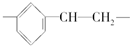 | ||
| C�� | �������ϩ�� | D�� | �۱�ϩ-CH2-CH2-CH2- |
18�������±�����ʵ�鼰����ó��Ľ��۲���ȷ���ǣ�������
| ʵ����Ŀ | ʵ������ |
| FeCl3��Һ�м���ά����C | ��Һ��ɫΪdz��ɫ |
| ����FeCl3��Һ�����ˮ�� | �õ����ɫҺ�� |
| ��ͭ����������� | ����ɫ���ݲ��� |
| A�� | ά����C���л�ԭ�� | B�� | FeCl3ˮ������Fe��OH��3���� | ||
| C�� | ͭ�������ᷴӦ��H2���� | D�� | ͭ���к���̼��� |
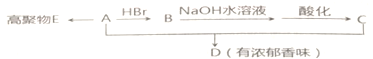
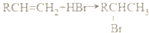
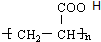 ��
��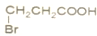 B��
B��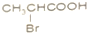 C��
C�� D��
D��