��Ŀ����
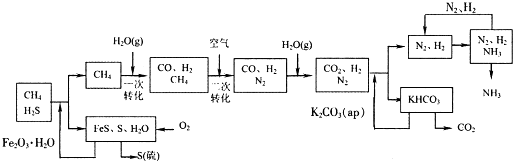
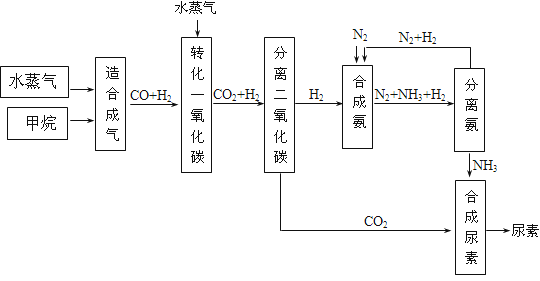
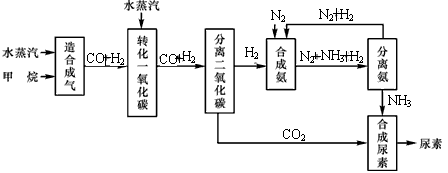
�Ĵ��зḻ����Ȼ����Դ������Ȼ��Ϊԭ�Ϻϳ����ص���Ҫ��������ͼ��ʾ��ͼ��ijЩת�����輰������δ�г�����

����д���пհף�
��1����֪0.5 mol�����0.5 molˮ������t �棬p k Paʱ����ȫ��Ӧ����һ����̼���������ϳ�������������a kJ�������÷�Ӧ���Ȼ�ѧ����ʽ�ǣ� _____________
��2���ںϳɰ���ʵ�����������У�����ȡ�Ĵ�ʩ֮һ�ǣ������ɵİ��ӻ�������м�ʹ����������������������ĵ���������ѭ�����ã�ͬʱ���䵪������������Ӧ�û�ѧ��Ӧ���ʺͻ�ѧƽ��Ĺ۵�˵����ȡ�ô�ʩ�����ɣ� _____________________
��3��������ϳɰ�����ת����Ϊ75��ʱ����5.60��107 L����Ϊԭ���ܹ��ϳ� L ������������������ڱ�״���²ⶨ��
��4����֪���صĽṹ��ʽΪ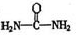 ����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ����
����
��
����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ����
����
��
��1��CH4(g)+H2O(g)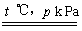 CO(g)+3H2(g)����H��2a kJ/mol
CO(g)+3H2(g)����H��2a kJ/mol
��2����������������Ũ������������Ӧ���ʣ���С������Ũ�ȣ���������������Ũ�ȶ�������ƽ��������Ӧ�����ƶ���
��3��1.12��108
��4��
����������1��������Ŀ��ʾд����ѧ��Ӧ����ʽ����ƽ��CH4+H2O��CO+3H2������ÿ��1mol�ļ����ˮ��Ӧ������2a kJ���������ٿ��Ǹ�����״̬����ɵ��Ȼ�ѧ����ʽ
��2����������������Ũ������������Ӧ���ʣ���С������Ũ�ȣ���������������Ũ�ȶ�������ƽ��������Ӧ�����ƶ�
��3���ɣ�CH4+H2O��CO+3H2��CO+ H2O=CO2+H2��3H2 + N2  2NH3
2NH3
�ۺϿɵã�3CH4��8NH3��������5.60��107 L����Ϊԭ���ܹ��ϳɰ���Ϊ��
V=5.60��107��75%��8/3=1.12��108L
��4�����ʵ��ƶ�̼��˫��λ�á���������ż��ɡ�

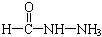
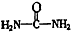 ����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ����__________________ ����_______________________ ��
����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ����__________________ ����_______________________ ��