��Ŀ����
���ʼ��仯�����ڹ�ҵ���������Ź㷺��Ӧ�á����������Ʊ��ߴ��ȵ����ᣨ����ṹʽΪ �������������ƣ��׳ơ����ơ����dz��õ�ˮ���������������ƣ�NaH2PO2�������ڻ�ѧ�����ȵȡ�
�������������ƣ��׳ơ����ơ����dz��õ�ˮ���������������ƣ�NaH2PO2�������ڻ�ѧ�����ȵȡ�
���������գ�
��1����Ԫ��ԭ�Ӻ������������Ų�ʽΪ ��NaH2PO2���漰������Ԫ�أ����ǵ�ԭ�Ӱ뾶��С�����˳��Ϊ ��
��2����ԭ�Ӻ����� �ֲ�ͬ�����ĵ��ӡ�
��3��д������ͬ���ڵ�����Ԫ���У����Ӱ뾶��С��Ԫ�أ�������������Ӧˮ����ĵ��뷽��ʽ ��
��4�������������Ҫ�����Ƹ������¯ˮ����ʳƷ��ҵ������ҵ�������������ˮ��Һ�����Ե�ԭ����_______________________________________________________��
��5�������������Ϊ�����������֮����ȥ����ˮ���ӵIJ����ṹʽΪ �����������ƣ��׳ơ����ơ����dz��õ�ˮ���������仯ѧʽΪ____________��
��6���������ƣ�NaH2PO2�������ڻ�ѧ��������ѧ��������Һ�к���Ni2+��H2PO2������һ���������ܷ������·�Ӧ��__Ni2++__H2PO2��+ ��__Ni + ___H2PO3��+ ������ɲ���ƽ������Ӧ���ӷ���ʽ���÷�Ӧ�Ļ�ԭ������__________��
��1��3S23P3 �� H<O<P<Na
��2��3
��3��H+ + AlO2- + H2O
 Al(OH)3
Al(OH)3
 Al3+
+ 3OH- ��2�֣�
Al3+
+ 3OH- ��2�֣�
��4�������������ӵ���̶ȴ���ˮ��̶� ��2�֣�
��5��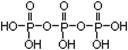 �� Na5P3O10
�� Na5P3O10
��6��Ni2++H2PO2��+H2O�� Ni+H2PO3��+2H+ ��2�֣� Ni
��������
�����������1��Hԭ����1�����Ӳ㣬O��2�����Ӳ㣬P��Na��3�����Ӳ���Na�������ٰ뾶�ʰ뾶��С˳��ΪH<O<P<Na��
��2����ԭ�ӵ����Ų�ʽΪ1S22S22P4��ͬ�������������ͬ����3�ֲ�ͬ�����ĵ��ӣ�
��3��H2PO2��Ϊ��������ӣ�����Һ�м���ˮ�����ܵ��룬ˮ��Һ�����Ե�ԭ��˵�������̶ȴ���ˮ��̶ȣ��Ե���Ϊ����
��4��������Ӽ���ˮӦ�������ǻ�����ˮ��Ӧ�����������ƣ��׳ơ����ơ������������������ǻ��ⱻ��ȡ���IJ��
��6������������ԭ��Ӧ�����غ���ƽ��������Ni2+���ɻ�ԭ����Ni��
���㣺���黯ѧ���������ʽṹ��������ԭ��Ӧ���й����⡣

 ��У����ϵ�д�
��У����ϵ�д�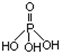 ���ʼ��仯�����ڹ�ҵ���������Ź㷺��Ӧ�ã����������Ʊ��ߴ��ȵ����ᣨ����ṹʽ��ͼ�������������ƣ��׳ơ����ơ����dz��õ�ˮ���������������ƣ�NaH2PO2�������ڻ�ѧ�����ȣ�
���ʼ��仯�����ڹ�ҵ���������Ź㷺��Ӧ�ã����������Ʊ��ߴ��ȵ����ᣨ����ṹʽ��ͼ�������������ƣ��׳ơ����ơ����dz��õ�ˮ���������������ƣ�NaH2PO2�������ڻ�ѧ�����ȣ�