��Ŀ����
ʵ������һƿ��������NaCl���ʵ�NaOH�����Լ���Ϊȷ���䴿�ȣ���������ζ������вⶨ ��
��
�ٳ�ȡWg NaOH�����Լ����Ƴ�100.00 mLˮ��Һ���ã�
�ڽ�Ũ��Ϊc mol/L�ı� ����װ���ñ�������ϴ����25.00 mL��ʽ�ζ����У�����Һ��λ��
����װ���ñ�������ϴ����25.00 mL��ʽ�ζ����У�����Һ��λ�� ����̶����£������¿̶ȣ�
����̶����£������¿̶ȣ�
��ȡV1 mL NaOH������Һ���ڽྻ����ƿ�У�����2��3�μ���ָʾ�������Ȼ����Ũ��Ϊc mol/L�ı�����ζ����ظ��ⶨ3�Σ�ƽ����ȥ����V2 mL��
�Իش�
��1�����Ʊ�������Һʱ������ʹ�õ�����Ҫ����������____________��
��2������1 mol/L��0.1 mol/L�ı�����Ӧѡ��_____mol/L���ᣬԭ����______________
________________________________ _____________________________��
_____________________________��
��3���ζ�ʱ���ζ�����������Ӧ��ע��_____________________���ζ��յ�ʱ��Һ��ɫ��_____ɫͻ��Ϊ____ɫ����ʢ�Ŵ�����Һ����ƿ�·���һ�Ű�ֽ�������� ��
��4�����в�����ʹʵ���ý��ƫ�����(��ѡ������) ��
E������ʱ�����ζ�ǰ���ӣ��ζ�����
��5�������Լ�NaOH�����������ı���ʽΪ ��
 ��
���ٳ�ȡWg NaOH�����Լ����Ƴ�100.00 mLˮ��Һ���ã�
�ڽ�Ũ��Ϊc mol/L�ı�
 ����װ���ñ�������ϴ����25.00 mL��ʽ�ζ����У�����Һ��λ��
����װ���ñ�������ϴ����25.00 mL��ʽ�ζ����У�����Һ��λ�� ����̶����£������¿̶ȣ�
����̶����£������¿̶ȣ���ȡV1 mL NaOH������Һ���ڽྻ����ƿ�У�����2��3�μ���ָʾ�������Ȼ����Ũ��Ϊc mol/L�ı�����ζ����ظ��ⶨ3�Σ�ƽ����ȥ����V2 mL��
�Իش�
��1�����Ʊ�������Һʱ������ʹ�õ�����Ҫ����������____________��
��2������1 mol/L��0.1 mol/L�ı�����Ӧѡ��_____mol/L���ᣬԭ����______________
________________________________
 _____________________________��
_____________________________����3���ζ�ʱ���ζ�����������Ӧ��ע��_____________________���ζ��յ�ʱ��Һ��ɫ��_____ɫͻ��Ϊ____ɫ����ʢ�Ŵ�����Һ����ƿ�·���һ�Ű�ֽ�������� ��
��4�����в�����ʹʵ���ý��ƫ�����(��ѡ������) ��
| A����ʪ���pH��ֽ�ⶨijNaOH��Һ��pH |
| B���к͵ζ�ʵ����������ˮϴ������ƿֱ��װ����Һ |
| C�����ζ�ǰ��ʽ�ζ��ܼ�������δ�ų����ζ�������������ʧ |
D��װ������� ��ʽ�ζ���û�н�����ϴ ��ʽ�ζ���û�н�����ϴ |
��5�������Լ�NaOH�����������ı���ʽΪ ��
1������ƿ
��2��0.1m/L,��ҺŨ��Խϡ�����ԽС��
��3����ƿ����Һ����ɫ�仯 ��ɫ ��ɫ�����ɫ�� ���ڹ۲���ƿ����Һ����ɫ�仯������ʵ�����
��4��CD
��5��
��2��0.1m/L,��ҺŨ��Խϡ�����ԽС��
��3����ƿ����Һ����ɫ�仯 ��ɫ ��ɫ�����ɫ�� ���ڹ۲���ƿ����Һ����ɫ�仯������ʵ�����
��4��CD
��5��

��

��ϰ��ϵ�д�
 ���б�ˢ��ϵ�д�
���б�ˢ��ϵ�д�
�����Ŀ

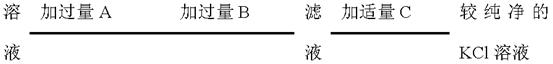

 ����ˮ�Ϳ�������Һ�е��κ�һ���Լ������ɼ����һ�������ǣ�������
����ˮ�Ϳ�������Һ�е��κ�һ���Լ������ɼ����һ�������ǣ������� ���־���
���־���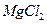 ��
��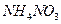 ������Һ
������Һ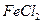 ��
��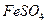 ������Һ
������Һ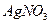 ��
��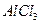 ������Һ
������Һ