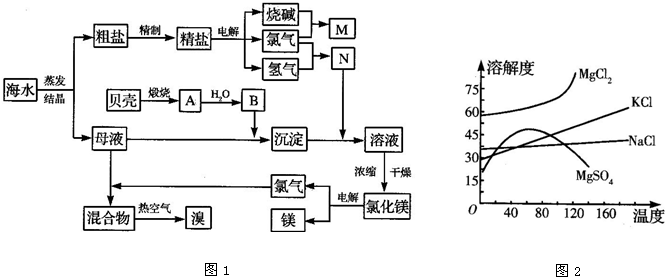
��Ŀ����
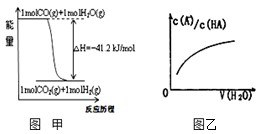 �������и�ͼ������������ǣ�������
�������и�ͼ������������ǣ�������| ��ѧ����ʽ | ƽ�ⳣ��K |
| F2+H2?2HF | 6.5��1095 |
| Cl2+H2?2HCl | 2.6��1033 |
| Br2+H2?2HBr | 1.9��1019 |
| I2+H2?2HI | 8.7��102 |
| A����֪1molCOȼ�շų�����Ϊ283kJ��2H2��g��+O2��g��=2H2O��g������H=-483.6kJ/mol��ͼ�ױ�ʾCO��H2O��g������CO2��H2�������仯 | ||
| B��������0.4 mol?L-1 HB��Һ��0.2 mol?L-1NaOH��Һ�������Ϻ���Һ��pH=3��������Һ������Ũ�ȵĴ�С˳��Ϊ��c��B-����c��Na+����c��H+����c��OH-�� | ||
| C�������£�X2��g����H2��Ӧ����HX��ƽ�ⳣ�������ʾ��������K�ı仯���Ϳ���˵������ͬ�����£�ƽ��ʱX2����F2��I2����ת�������С����HX�Ļ�ԭ������ | ||
D��ͼ�������߱�ʾ������������HA��ϡ��Һ�м�ˮϡ�����У�
|
������A������CO��ȼ������д�Ȼ�ѧ����ʽ���������ȼ�յ��Ȼ�ѧ����ʽ�����ø�˹���ɼ���CO��H2O��g������CO2��H2�ķ�Ӧ�ȣ���ͼ��ȽϽ��
B.0.4 mol?L-1 HB��Һ��0.2 mol?L-1NaOH��Һ�������Ϻ�ΪHB��NaB��Ũ�ȶ���0.2mol/L�Ļ����Һ����Һ��pH=3��˵��HBΪ���ᣬHB�ĵ���̶ȴ���B-��ˮ��̶ȣ���Һ�����ԣ��ݴ��жϣ�
C��ƽ�ⳣ��Խ��Ӧ���еij̶�Խ��ת��������ʼ���ʵ�Ũ���йأ�ƽ�ⳣ�����������أ�
D��������HA��ϡ��Һ�м�ˮϡ�ͣ�����̶�������Һ��n��A-������n��HA����С���ݴ��жϣ�
B.0.4 mol?L-1 HB��Һ��0.2 mol?L-1NaOH��Һ�������Ϻ�ΪHB��NaB��Ũ�ȶ���0.2mol/L�Ļ����Һ����Һ��pH=3��˵��HBΪ���ᣬHB�ĵ���̶ȴ���B-��ˮ��̶ȣ���Һ�����ԣ��ݴ��жϣ�
C��ƽ�ⳣ��Խ��Ӧ���еij̶�Խ��ת��������ʼ���ʵ�Ũ���йأ�ƽ�ⳣ�����������أ�
D��������HA��ϡ��Һ�м�ˮϡ�ͣ�����̶�������Һ��n��A-������n��HA����С���ݴ��жϣ�
����⣺A��CO��ȼ������д�Ȼ�ѧ����ʽΪCO��g��+
O2��g��=CO2��g����H=-283kJ/mol ��
2H2��g��+O2��g��=2H2O��g������H=-483.6kJ/mol �ڣ�
��-�ڡ�
��CO��g��+H2O��g��=CO2��g��+H2��g������H=-41.2kJ/mol����ͼ����ϣ���A��ȷ��
B.0.4 mol?L-1 HB��Һ��0.2 mol?L-1NaOH��Һ�������Ϻ�ΪHB��NaB��Ũ�ȶ���0.2mol/L�Ļ����Һ����Һ��pH=3��˵��HBΪ���ᣬHB�ĵ���̶ȴ���B-��ˮ��̶ȣ���Һ�����ԣ���c��B-����c��Na+����c��H+����c��OH-����������ʵĵ���̶Ȳ���c��B-����c��Na+����c��H+����c��OH-������B��ȷ��
C��ƽ�ⳣ��Խ��Ӧ���еij̶�Խ��ת��������ʼ���ʵ�Ũ���йأ�ƽ�ⳣ����ijһ��Ӧ���ת���ʲ�һ����ƽ�ⳣ�����������أ���C����
D��������HA��ϡ��Һ�м�ˮϡ�ͣ�����̶�������Һ��n��A-������n��HA����С����
��ֵ����ͼ����ʵ�ʷ��ϣ���D��ȷ��
��ѡC��
| 1 |
| 2 |
2H2��g��+O2��g��=2H2O��g������H=-483.6kJ/mol �ڣ�
��-�ڡ�
| 1 |
| 2 |
B.0.4 mol?L-1 HB��Һ��0.2 mol?L-1NaOH��Һ�������Ϻ�ΪHB��NaB��Ũ�ȶ���0.2mol/L�Ļ����Һ����Һ��pH=3��˵��HBΪ���ᣬHB�ĵ���̶ȴ���B-��ˮ��̶ȣ���Һ�����ԣ���c��B-����c��Na+����c��H+����c��OH-����������ʵĵ���̶Ȳ���c��B-����c��Na+����c��H+����c��OH-������B��ȷ��
C��ƽ�ⳣ��Խ��Ӧ���еij̶�Խ��ת��������ʼ���ʵ�Ũ���йأ�ƽ�ⳣ����ijһ��Ӧ���ת���ʲ�һ����ƽ�ⳣ�����������أ���C����
D��������HA��ϡ��Һ�м�ˮϡ�ͣ�����̶�������Һ��n��A-������n��HA����С����
| c(A-) |
| c(HA) |
��ѡC��
���������⿼�黯ѧ��Ӧ�������仯������Ũ�ȱȽϡ�ƽ�ⳣ�������塢������ʵĵ���ȣ���Ŀ��Ϊ�ۺϣ��Ѷ��еȣ�Cѡ��Ϊ�״��㣬ע��ƽ�ⳣ�������壮

��ϰ��ϵ�д�
�����Ŀ
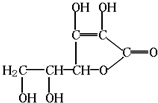 ά����C��һ��ˮ����ά���أ���ˮ��Һ�����ԣ���ѧʽΪC6H8O6���ṹ��ͼ��ʾ������ȱ������ά����C�û�Ѫ֢������ά����C�ֳƿ���Ѫ�ᣮά����C�ױ������е����������������ʵ�ˮ�����߲ˡ�����Ʒ�ж���ά����C�������ʵij�֭��ά����C�ĺ�����500mg/L���ң����й���ά����C������������ǣ�������
ά����C��һ��ˮ����ά���أ���ˮ��Һ�����ԣ���ѧʽΪC6H8O6���ṹ��ͼ��ʾ������ȱ������ά����C�û�Ѫ֢������ά����C�ֳƿ���Ѫ�ᣮά����C�ױ������е����������������ʵ�ˮ�����߲ˡ�����Ʒ�ж���ά����C�������ʵij�֭��ά����C�ĺ�����500mg/L���ң����й���ά����C������������ǣ�������| A��ά����C��ʹ��ˮ��ɫ | B��ά����C����ʳƷ���Ӽ� | C��ά����C�ɷ���ˮ�ⷴӦ | D��ά����C���ܷ���������Ӧ |
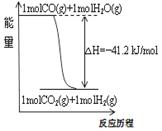
�������и�ͼ������������� (����)
 |

| ��ѧ����ʽ | ƽ�ⳣ��K |
| F2+H2 | 6.5��1095 |
| Cl2+H2 | 2.6��1033 |
| Br2+H2 | 1.9��1019 |
| I2 + H2 | 8.7��102 |
ͼ �� �� �� ͼ ��
A����֪1molCOȼ�շų�����Ϊ283kJ��2H2(g)+O2(g)=2H2O(g)����H= - 483.6kJ/mol��ͼ�ױ�ʾCO��H2O(g)����CO2��H2�������仯
B��������0��4 mol��L��1 HB��Һ��0��2 mol��L��1NaOH��Һ�������Ϻ���Һ��pH=3��������Һ������Ũ�ȵĴ�С˳��Ϊ��c��B-��> c��Na+��>c��H+��>c��OH-��
C�������£�X2(g)��H2��Ӧ����HX��ƽ�ⳣ���������ʾ��������K�ı仯���Ϳ���˵������ͬ�����£�ƽ��ʱX2����F2��I2����ת�������С����HX�Ļ�ԭ������
D��ͼ�������߱�ʾ������������HA��ϡ��Һ�м�ˮϡ�����У�![]() �ı仯���
�ı仯���