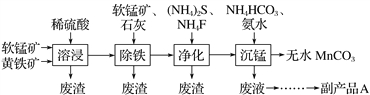
��Ŀ����
����Ŀ���о�CO2�ں����е�ת�ƺ��ޣ��ǵ������ѧ�о���ǰ������
��1�����ں�ˮ�� CO2��Ҫ��4����̼��ʽ���ڣ����� HCO3��ռ95%��д��CO2����ˮ����HCO3���ķ���ʽ��___________��
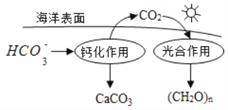
��2���ں���ѭ���У�ͨ����ͼ��ʾ��;����̼�� д���ƻ����õ����ӷ���ʽ__________��
��3����ˮ���ܽ���̼ռ��ˮ��̼�� 95%���ϣ���ȷ�������о�����̼ѭ���Ļ����������ܽ���̼���ɲ������·����� �����ᡢ����CO2����N2���ữ��ĺ�ˮ�д��� CO2 ���ü�Һ���գ�װ����ͼ���������߿��е�װ�ò�����������������Լ�_________
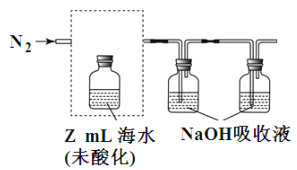
���ζ���������Һ���յ���̼ת��ΪNaHCO3������x mol/L����ζ�������y mL���ᣬ��ˮ���ܽ���̼��Ũ��=________mol/L��
��4��������ͼ��ʾװ�ôӺ�ˮ����ȡ CO2�������ڼ��ٻ����������庬����
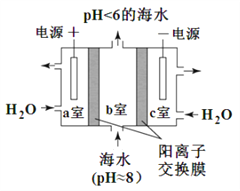
�� ��Ϸ���ʽ������ȡ CO2��ԭ����_____________��
���ø�װ�ò��������ʴ��� b���ų��ĺ�ˮ���ϸ���Żش��������ϸ�ľ��巽����__________��
���𰸡� CO2��H2O![]() H2CO3�� H2CO3
H2CO3�� H2CO3![]() HCO3����H+ Ca2+��2HCO3��= CaCO3����CO2����H2O
HCO3����H+ Ca2+��2HCO3��= CaCO3����CO2����H2O 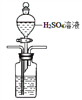 xy/2 a �ң�2H2O��4e��=O2����4H+��H+ͨ��������Ĥ��a�ҽ���b�ң�������Ӧ��HCO3����H+=CO2��+H2O �� c ���ų��ļ�Һ����b���ų������Ժ�ˮ�������ӽ�װ����ں�ˮ�� pH
xy/2 a �ң�2H2O��4e��=O2����4H+��H+ͨ��������Ĥ��a�ҽ���b�ң�������Ӧ��HCO3����H+=CO2��+H2O �� c ���ų��ļ�Һ����b���ų������Ժ�ˮ�������ӽ�װ����ں�ˮ�� pH
����������1��������̼����ˮ���ɵ�̼��Ϊ���ᣬ���ֵ�������̼��������йط���ʽΪ��CO2+H2OH2CO3��H2CO3H++HCO3-��
��2����Ӧ���к���̼�������������Ϊ̼��ƣ�����Ԫ���غ��Լ�����غ�ó�����ʽΪ��2HCO3-+Ca2+=CaCO3��+CO2��+H2O��
��3�����ữ��ˮ������ʹ���Լ���ϡ���ᣬ���÷�Һ©���μӣ����ܽ������̹ܳ�������װ��Ϊ��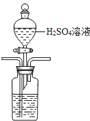 ��
��
�ڴ˷�Ӧԭ��Ϊ��NaHCO3+HCl=NaCl+CO2��+H2O����̼����������������ʵ���֮��Ϊ1��1����ô��ˮ��̼�����Ƶ�Ũ��Ϊc�������ΪmL������������c��z=xy����c=![]() ��
��
��4����a�ң�2H2O-4e-=4H++O2����������ͨ�������ӽ���Ĥ����b�ң�������Ӧ��H++HCO3-=CO2��+H2O��
���ø�װ�ò��������ʴ���b���ų��ĺ�ˮ���ϸ���Żش��������ϸ�ķ����ǣ�c�ң�2H2O+2e-=2OH-+H2������c���ų��ļ�Һ����b���ų������Ժ�ˮ������װ����ں�ˮ��pH��

 ��У����ϵ�д�
��У����ϵ�д�