��Ŀ����
��������(SnSO4)��һ����Ҫ��������ˮ�������Σ��㷺Ӧ���ڶ�����ҵ��ij�о�С��
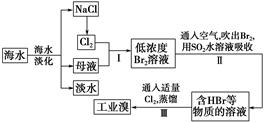
���SnSO4�Ʊ�·�����£�
�������ϣ�
I�����������£�����ˮ��Һ����Sn2+��Sn4+������Ҫ������ʽ��Sn2+�ױ�������
��SnC12��ˮ�����ɼ�ʽ�Ȼ�����[Sn(OH)Cl]
�ش��������⣺
��1������l�IJ���Ϊ ____��____�����ˡ�ϴ�ӡ�����Գ�������ϴ�ӵķ�����_____________��
��2�� SnCl2��ĩ���Ũ��������ܽ⣬���ϱ�Ҫ�Ļ�ѧ����ʽ��ƽ���ƶ�ԭ������ԭ��________��
��3������Sn�۵��������������ٵ�����ҺpH;��__________��
��4�����������£�SnSO4����������˫��ˮȥ������������Ӧ�����ӷ���ʽ�ǣ�________________��
��5����С��ͨ�����з����ⶨ�������۵Ĵ��ȣ����ʲ����뷴Ӧ����ȡag�������������У������ɵ�SnC12�м��������FeC13��Һ����b mol/LK2Cr2O7�ζ����ɵ�Fe2+����֪���Ի����£�Cr2O72-�ɱ���ԭΪCr3+��������ȥK2Cr2O7��Һm ml������������������������________����Sn��Ħ������ΪM g/mol���ú�a��b��m��M�Ĵ���ʽ��ʾ��
��1������Ũ������ȴ�ᾧ������������©���У�������ˮ��û������ʹˮ��Ȼ���£��ظ�����2-3��
��2��SnCl2 + H2O Sn(OH)Cl + HCl���������ᣬʹ��ƽ�������ƶ�������Sn2+ˮ��
Sn(OH)Cl + HCl���������ᣬʹ��ƽ�������ƶ�������Sn2+ˮ��
��3����ֹSn2+������
��4��Sn2+ + H2O2 +2H+ = Sn4+ + 2H2O
��5��3bmM /1000a
���������������1������SnSO4���ܽ�����¶ȵ�Ӱ��仯�ϴ����¶ȵ����߶��������¶ȵĽ��Ͷ���С�����Դ�SnSO4��Һ�л��SnSO4����ķ���������Ũ������ȴ�ᾧ��ϴ�ӳ����ķ����ǽ���������©���У�������ˮ��û������ʹˮ��Ȼ���£��ظ�����2-3�Ρ���2�� SnCl2��ĩ���Ũ��������ܽ⣬����ΪSnCl2��ǿ�������Σ���ˮ��������ˮ�ⷴӦSnCl2 + H2O Sn(OH)Cl + HCl������HCl���ܽ⣬�����������������Ũ�ȣ�����ʹˮ��ƽ�����淴Ӧ�����ƶ���������SnCl2��ˮ�ⷴӦ�ķ�������3�� ���������������£�����ˮ��Һ����Sn2+��Sn4+������Ҫ������ʽ��Sn2+�ױ����������Լ���Sn�۵��������������ٵ�����ҺpH;�ڷ�ֹSn2+����������5���йط�Ӧ�ķ���ʽΪSn+2HCl=SnCl2+H2����SnCl2+2FeC13=SnCl4+2FeC12�� 6Fe2++Cr2O72-+14H+=6Fe3++2Cr3++7H2O���ɷ���ʽ�ù�ϵʽΪ3Sn����6 FeC12����K2Cr2O7��n(Cr2O72-)=mb��10-3mol.����n(Sn)=3mb��10-3mol.������������������������(3mb��10-3mol��Mg/mol)��ag="3bmM" /1000a.
Sn(OH)Cl + HCl������HCl���ܽ⣬�����������������Ũ�ȣ�����ʹˮ��ƽ�����淴Ӧ�����ƶ���������SnCl2��ˮ�ⷴӦ�ķ�������3�� ���������������£�����ˮ��Һ����Sn2+��Sn4+������Ҫ������ʽ��Sn2+�ױ����������Լ���Sn�۵��������������ٵ�����ҺpH;�ڷ�ֹSn2+����������5���йط�Ӧ�ķ���ʽΪSn+2HCl=SnCl2+H2����SnCl2+2FeC13=SnCl4+2FeC12�� 6Fe2++Cr2O72-+14H+=6Fe3++2Cr3++7H2O���ɷ���ʽ�ù�ϵʽΪ3Sn����6 FeC12����K2Cr2O7��n(Cr2O72-)=mb��10-3mol.����n(Sn)=3mb��10-3mol.������������������������(3mb��10-3mol��Mg/mol)��ag="3bmM" /1000a.
���㣺�������������ϴ�ӳ����ķ������ε�ˮ��ƽ���ƶ�������ʽ����д����ϵʽ���ڼ������ʴ��ȵļ����Ӧ�õ�֪ʶ��

 �ο�������ϵ�д�
�ο�������ϵ�д� ������ѧ��ʱ��ҵϵ�д�
������ѧ��ʱ��ҵϵ�д� ���������ʱ��ѵϵ�д�
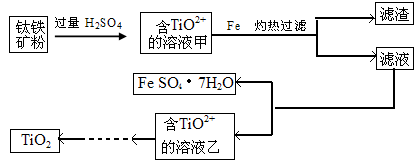
���������ʱ��ѵϵ�д�����������(��Ҫ��Fe2O3��SiO2��Al2O3��MgO������)�Ʊ��������Ĺ����������£�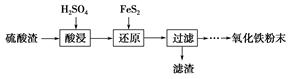
(1)�������������Ҫ�ʵ�������Ŀ���ǣ���������Ľ����ʣ���________��
(2)����ԭ���ǽ�Fe3��ת��ΪFe2����ͬʱFeS2������ΪSO42�����÷�Ӧ�����ӷ���ʽΪ________________________________________________________________________��
(3)Ϊ�ⶨ��������������Һ��Fe3�������Կ��Ƽ���FeS2������ʵ�鲽��Ϊ��ȷ��ȡһ���������������Һ����ƿ�У�����ϡ���ᡢ�Թ���SnCl2���ټ�HgCl2��ȥ������SnCl2���Զ�����������Ϊָʾ������K2Cr2O7����Һ�ζ����йط�Ӧ�Ļ�ѧ����ʽ���£�
2Fe3����Sn2����6Cl��=2Fe2����SnCl62��
Sn2����4Cl����2HgCl2=SnCl62����Hg2Cl2��
6Fe2����Cr2O72����14H��=6Fe3����2Cr3����7H2O
����SnCl2����������ⶨ��Fe3����________(�ƫ�ߡ�����ƫ�͡����䡱����ͬ)��
��������HgCl2����ⶨ��Fe3����________��
(4)�ٿ�ѡ��________(���Լ�)������Һ�к���Fe3��������Fe3����ԭ����________________________________________________________________________(�����ӷ���ʽ��ʾ)��
����֪����������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Al(OH)3 | Fe(OH)2 | Mg(OH)2 | Mn(OH)2 |
| ��ʼ���� | 2.7 | 3.8 | 7.5 | 9.4 | 8.3 |
| ��ȫ���� | 3.2 | 5.2 | 9.7 | 12.4 | 9.8 |
ʵ���ѡ�õ��Լ��У�ϡ���ᡢBa(NO3)2��Һ������KMnO4��Һ��NaOH��Һ��Ҫ���Ʊ������в������ж����塣
������ɡ����ˡ������Һģ���Ʊ���������ʵ�鲽�裺
a��������________________________________________________________��
b��������__________________________________________________________��
c�����룬ϴ�ӣ�
d����ɣ���ĥ��