��Ŀ����
����Ŀ��̼���̣�MnCO3��������ĸ�����ǿ���Բ��ϣ�Ҳ���Ʊ�Mn2O3��MnO2���̵����������Ҫԭ�ϣ��㷺���ڵ��ӡ�������ҽҩ����ҵ��
��1����ҵ���Ʊ�����ʽΪ��MnSO4+2NH4HCO3=MnCO3��+��NH4��2SO4+CO2��+H2O����Ӧ��ͨ��������Թ�����NH4HCO3���ҿ�����Һ��pHΪ6.8��7.4�������Թ�����NH4HCO3��Ŀ���� ��
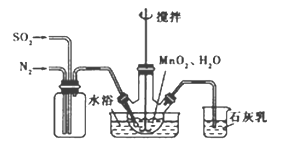
��2��ʵ����ģ�ҵ���������Ʊ�������װ����ͼ
��ʯ������뷴Ӧ�Ļ�ѧ����ʽΪ ��
����Ӧ�����У�ΪʹSO2������ת����ȫ����ͨ��SO2��N2����һ�������ı��ҺͶ�ϵ������£��ɲ�ȡ�ĺ�����ʩ�� ��
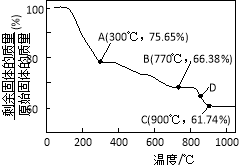
��3��MnCO3�ڿ����м�����ת��Ϊ��ͬ��̬���̵�������������������¶ȵı仯��ͼ��ʾ����300��ʱ��ʣ�������n(Mn����n(O��Ϊ ��ͼ�е�D��Ӧ����ijɷ�Ϊ ���ѧʽ����
���𰸡���1�������Թ�����NH4HCO3�����MnSO4��ת������ʹMnCO3������ȫ
��2����Ca(OH��2ʮSO2=CaSO3+H2O��
�������ʵ����¶ȡ�����ͨ�˻������
��1��2 (3���� Mn3O4��MnO(����������������
��������
�����������1��MnSO4+2NH4HCO3=MnCO3��+��NH4��2SO4+CO2��+H2O����Ӧ��ͨ��������Թ�����NH4HCO3���ҿ�����Һ��pHΪ6.8��7.4�������Թ�����NH4HCO3��Ŀ�������MnSO4��ת���ʣ�ʹMnCO3������ȫ��
��2�������Ʊ�������װ��ͼ��
��ʯ�����Ƿ�ֹ����������Ⱦ���������뷴Ӧ�Ļ�ѧ����ʽΪCa(OH��2ʮSO2=CaSO3+H2O��
����Ӧ�����У�ΪʹSO2������ת����ȫ����ͨ��SO2��N2����һ�������ı��ҺͶ�ϵ������£��ɲ�ȡ�ĺ�����ʩ�п����ʵ����¶ȡ�����ͨ�˻��������
��3������MnCO3�����ʵ���Ϊ1 mol��������Ϊ115 g
��A��ʣ���������Ϊ115 g��75.65%��87 g
���ٵ�����Ϊ115 g��87 g��28 g
��֪MnCO3ʧȥ�����ΪCO
��ʣ�����ijɷ�ΪMnO2
��C��ʣ���������Ϊ115 g��61.74%��71 g
����Ԫ���غ�֪m(Mn����55 g����m(O ��1��71 g��55 g��16 g
��n(Mn����n(O����![]() ��
��![]() ��1��1
��1��1
��ʣ�����ijɷ�ΪMnO
ͬ����B��ʣ���������Ϊ115 g��66.38%��76.337 g
��m(Mn����55 g����m(O ��2��76.337 g��55 g��21.337 g
��n(Mn����n(O����![]() ��
��![]() ��3��4
��3��4
��ʣ�����ijɷ�ΪMn3O4
��D�����B��C֮�䣬��D���Ӧ����ijɷ�ΪMn3O4��MnO�Ļ������