��Ŀ����
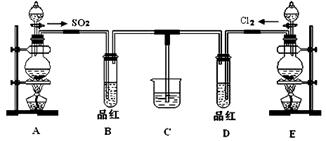
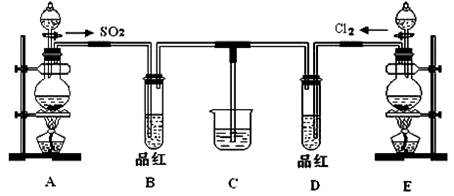
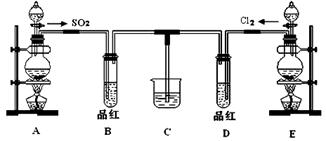
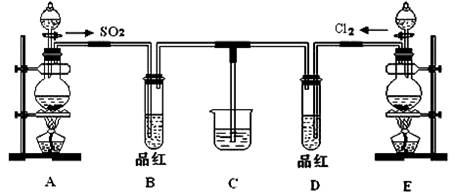
ij��ѧʵ��С��ͬѧΪ��֤�����Ƚ�SO2����ˮ��Ư���ԣ����������ͼ��ʾ��ʵ��װ�á�
��1��ʵ���ҳ�ѡ����CO2�ķ���װ����SO2��ʵ������MnO2��Ũ���ᷴӦ�Ʊ�Cl2ʱ��Ӧѡ����ͼA��E������װ���� װ�ã���װ����ţ���Cl2��ͨ�� ����д�������ƣ�����ƿ�м���������Ũ���ᣬ��Ӧ���ӷ���ʽΪ
��
��2����Ӧ��ʼ����B��D�����Թ��е�Ʒ����Һ����ȥ��ֹͣͨ����B��D�����Թܼ��ȣ������Թ��е�����ֱ�Ϊ
B�� ��
D�� ��
��3����������ͬѧ�ֱ�������ͼ��ʾװ��̽���������尴��ͬ������Ϻ��Ư���ԡ�
��ͬѧ��ʵ������з��֣�ͨ��һ��ʱ���Ʒ����Һ��������ɫ����ԭ���ǣ��û�ѧ��Ӧ����ʽ��ʾ����
��1��E ��Һ©�� MnO2+4H++2Cl![]()
![]() Mn
Mn![]() +Cl2
+Cl2![]() +2H2O
+2H2O
��2��B����Һ����ɫ��ɺ�ɫ D����Һû�����Եı仯
��3��SO2+Cl2+2H2O==2HCl+H2SO4
����:��