��Ŀ����
����Ŀ���״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧԭ����
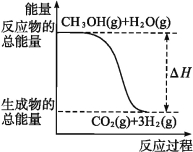
��CH3OH(g)+H2O(g)![]() CO2(g)+3H2(g) ��H=+49��0 kJ��mol-1
CO2(g)+3H2(g) ��H=+49��0 kJ��mol-1
��CH3OH(g)+ ![]() O2(g)
O2(g)![]() CO2(g)+2H2(g) ��H=-192��9 kJ��mol-1
CO2(g)+2H2(g) ��H=-192��9 kJ��mol-1
����˵����ȷ����
A��CH3OH��ȼ���ȵĦ�H=-192.9 kJ��mol-1
B����Ӧ���е������仯����ͼ��ʾ
C��CH3OHת���H2�Ĺ���һ��Ҫ��������
D����������֪��ӦCH3OH(l)+![]() O2(g)
O2(g)![]() CO2(g)+2H2(g)�Ħ�H>-192.9 kJ��mol-1
CO2(g)+2H2(g)�Ħ�H>-192.9 kJ��mol-1
���𰸡�D
����������Ӧ����H2(g)�����ȶ�����������A���ȷ����Ӧ��Ϊ���ȷ�Ӧ����ͼʾ��ӦΪ���ȷ�Ӧ��B���ȷ���������Ӧ��������֪��CH3OHת���H2�Ĺ��̿�������������Ҳ���Էų�������C���ȷ������CH3OH����̬ʱ����Һ̬ʱ����������֪����CH3OH(l)��CH3OH(g)��Ӧʱ�ų�����������D����ȷ��

��ϰ��ϵ�д�
 ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
�����Ŀ