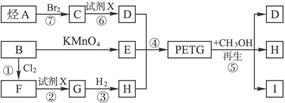
��Ŀ����
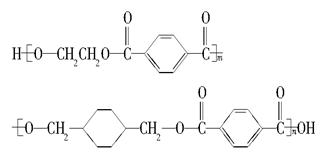
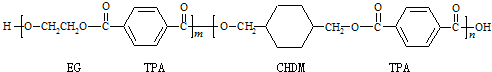
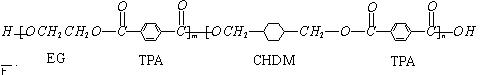
�ҹ��ڶ�������֤���õ��Ǿ�����ɫ�������ܵ�PETG�²��ϣ�PETG�²��Ͽ��Ի��������ã����Ҷ��ܱ����������κ���Ⱦ����һ�������ɽ��ջ�����ҵ��չ����˾�����з��ɹ����²��ϣ�����Ϊ������������أ�PETG�Ľṹ��ʽΪ��
��֪����
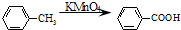
��RCOOR1+R2OH��RCOOR2+R1OH ��R��R1��R2��ʾ���������ֲ��Ͽɲ������кϳ�·��
�Իش��������⣺
��1����Ӧ�ڡ����м�����Լ�X��______��
��2���ݵķ�Ӧ������______��
��3��д���ṹ��ʽ��B______��I______��
��4���ϳ�ʱӦ���Ƶĵ�������ʵ���n��D����n��E����n��H��=______��______��______����m��n��ʾ����
��5��д����ѧ����ʽ��
��Ӧ�ۣ�______����Ӧ�ޣ�______��

��֪����
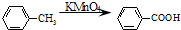
��RCOOR1+R2OH��RCOOR2+R1OH ��R��R1��R2��ʾ���������ֲ��Ͽɲ������кϳ�·��

�Իش��������⣺
��1����Ӧ�ڡ����м�����Լ�X��______��
��2���ݵķ�Ӧ������______��
��3��д���ṹ��ʽ��B______��I______��
��4���ϳ�ʱӦ���Ƶĵ�������ʵ���n��D����n��E����n��H��=______��______��______����m��n��ʾ����
��5��д����ѧ����ʽ��
��Ӧ�ۣ�______����Ӧ�ޣ�______��
����PETG�Ľṹ��ʽ���Կ���PETG����

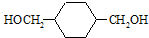
��HOCH2CH2OH��

���ֵ���ͨ�����۷�Ӧ�õ���һ�ָ߾��
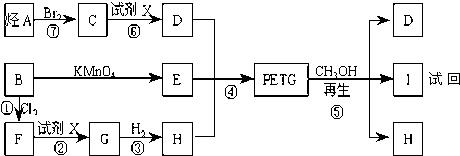
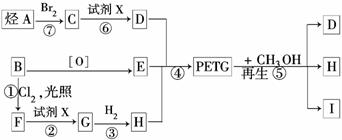
PETG�ͼ״���Ӧ����D��I��H������RCOOR1+R2OH��RCOOR2+R1OH֪��D��H�Ǵ�����E��

��B������ط�Ӧ����

������B��

��

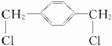
����������ȡ����Ӧ����F
��

��X��Ӧ����G��G��Ӧ����H������H��
����G��

��
����D���Ҷ�����B����ϩ����ϩ���巢���ӳɷ�Ӧ����C 1��2�������飬PETG�ͼ״���Ӧ����D��I��H����������Ϣ֪��I��
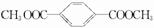
��
��1��ͨ�����Ϸ���֪��±�������������Ƶ�ˮ��Һ�������ɴ�������X��NaOH��Һ���ʴ�Ϊ������������Һ��
��2��PETG�ͼ״���Ӧ����D��I��H�ķ�Ӧ����ȡ����Ӧ���ʴ�Ϊ��ȡ����Ӧ��
��3��ͨ�����Ϸ���֪��B��I�Ľṹ��ʽ�ֱ�Ϊ��
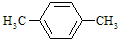
��
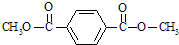
��
�ʴ�Ϊ��

��

��
��4���ɾۺ����֪���ϳ�ʱӦ���Ƶĵ�������ʵ�����n��D����n��E����n��H��=m����m+n����n���ʴ�Ϊ��m����m+n����n��
��5��ͨ�����Ϸ���֪���۵ķ�Ӧ����ʽΪ��
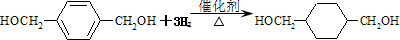
��
�ķ�Ӧ����ʽΪ��CH2Br-CH2Br+2H2O
HOCH2-CH2OH+2HBr��
�ʴ�Ϊ��
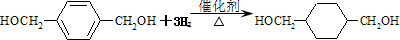
��
�ķ�Ӧ����ʽΪ��CH2Br-CH2Br+2H2O
HOCH2-CH2OH+2HBr��

��HOCH2CH2OH��

���ֵ���ͨ�����۷�Ӧ�õ���һ�ָ߾��
PETG�ͼ״���Ӧ����D��I��H������RCOOR1+R2OH��RCOOR2+R1OH֪��D��H�Ǵ�����E��

��B������ط�Ӧ����

������B��

��

����������ȡ����Ӧ����F

��

��X��Ӧ����G��G��Ӧ����H������H��

����G��

��
����D���Ҷ�����B����ϩ����ϩ���巢���ӳɷ�Ӧ����C 1��2�������飬PETG�ͼ״���Ӧ����D��I��H����������Ϣ֪��I��

��
��1��ͨ�����Ϸ���֪��±�������������Ƶ�ˮ��Һ�������ɴ�������X��NaOH��Һ���ʴ�Ϊ������������Һ��
��2��PETG�ͼ״���Ӧ����D��I��H�ķ�Ӧ����ȡ����Ӧ���ʴ�Ϊ��ȡ����Ӧ��
��3��ͨ�����Ϸ���֪��B��I�Ľṹ��ʽ�ֱ�Ϊ��

��

��
�ʴ�Ϊ��

��

��
��4���ɾۺ����֪���ϳ�ʱӦ���Ƶĵ�������ʵ�����n��D����n��E����n��H��=m����m+n����n���ʴ�Ϊ��m����m+n����n��
��5��ͨ�����Ϸ���֪���۵ķ�Ӧ����ʽΪ��
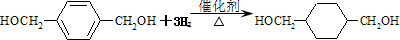
��
�ķ�Ӧ����ʽΪ��CH2Br-CH2Br+2H2O
| NaOH |
| �� |
�ʴ�Ϊ��
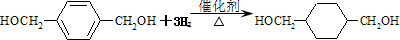
��
�ķ�Ӧ����ʽΪ��CH2Br-CH2Br+2H2O
| NaOH |
| �� |

��ϰ��ϵ�д�
 ������ҵ����ν�����������ϵ�д�
������ҵ����ν�����������ϵ�д�
�����Ŀ
�ҹ��ڶ�������֤���õ��Ǿ�����ɫ�������ܵ�PETG�²��ϣ�PETG�²�
�Ͽ��Ի��������ã����Ҷ��ܱ����������κ���Ⱦ��PETG�Ľṹ��ʽΪ��
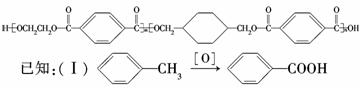 |
(��)RCOOR�䣫R��OH�D��RCOOR�士R��OH(R��R��R���ʾ����)
 ���ֲ��Ͽɲ�������·�ߺϳɣ�
���ֲ��Ͽɲ�������·�ߺϳɣ�
�Իش��������⣺
(1)��Ӧ�ڢ�����Լ�X��________����Ӧ�١���������ȡ����Ӧ����________��
(2)д������I�Ľṹ��ʽ��____________________________________________.
(3)д����Ӧ�Ļ�ѧ����ʽ��___________________________________________
______________________________________________________________________.
(4)�ϳ�PETGʱ����������ʵ����ı�����ϵ�ǣ�
n(D)��n(E)��n(H)��________(��m��n��ʾ)��








 RCOOR2+R1OH��R��R1��R2����������
RCOOR2+R1OH��R��R1��R2����������