��Ŀ����
������һ�������ԵĻ��⣬��γ��������������������Դ��Ϊ��������ǹ��Ƽ������߲�иŬ����Ŀ�ꡣ
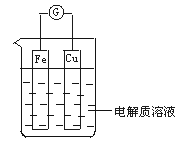
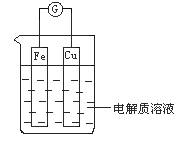
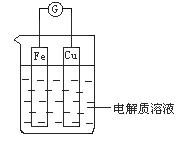
��1����ͼ��ʾ�����һ��ԭ��أ�
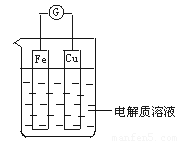
�ٵ��������ҺΪϡ����ʱ��
Cu�缫��_____(���������)������缫��ӦΪ____���÷�Ӧ��____(���������ԭ��)��Ӧ��
�ڵ��������ҺΪŨ����ʱ��
Cu�缫��_____������缫��ӦΪ__________���÷�Ӧ��_____��Ӧ��
��2������ǽ� ��ת��Ϊ �ܡ���д���������ͭ��Һ���ܻ�ѧ����ʽ____________
��3��ȼ������ʱ������С����������.��֪4��H2ȼ������Һ̬ˮʱ����Ϊ571.6kJ����д����ʾH2ȼ���ȵ��Ȼ�ѧ����ʽΪ�� .
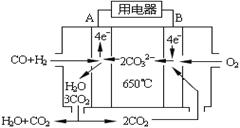
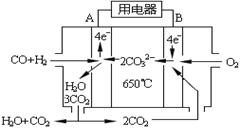
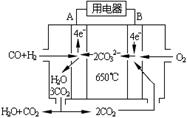
��4����ͼ��һ̼����ȼ�ϵ�أ�MCFC������ˮú����CO��H2��Ϊȼ�ϣ�һ������Li2CO3��Na2CO3���ۻ����Ϊ����ʡ�д��B�������ĵ缫��Ӧʽ ��
���𰸡�
�Ţ� �� ����1�֣�
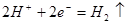 ����2�֣� ��ԭ ����1�֣�
����2�֣� ��ԭ ����1�֣�
�ڸ� ����1�֣� 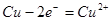 �� ��2�֣�����
����1�֣�
�� ��2�֣�����
����1�֣�
�� �� ����ѧ ����ÿ��1�֣�

�� 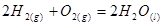
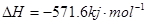 ��2�֣�
��2�֣�
�� 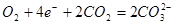
����������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ