��Ŀ����
��763K��3.04��104kPaʱ����CO��H2��ԭ�Ϻϳ�CH3OH����������ƽ�⣺CO��g��+2H2��g�� CH3OH��g������ԭ����CO��H2�ı�����ͬʱ����CO��ת���ʼ�ƽ�������м״��������������Ӱ�졣
CH3OH��g������ԭ����CO��H2�ı�����ͬʱ����CO��ת���ʼ�ƽ�������м״��������������Ӱ�졣��1����H2��CO��ʼ���ʵ���֮��Ϊm��ƽ��ʱCO��ת����Ϊ����ƽ�������м״����������Ϊy����m������y���ߵĹ�ϵʽΪ________________��
��2�����±���֪���ݴ���������ϵʽ�н��м��㣬��������������еĿո����١��ڡ����У���

��3�����ݱ����ṩ�����ݣ��ɵó���Ӧ��ı�����COת�����Լ�ƽ�������м״����������Ӱ��Ľ��ۣ���ѡ����ѷ�Ӧ����ȡ�
��1��y=
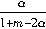 ��
����2����16.67%�� ��21.43%�� ��0.56
��3���ɱ������ݿ�֪��mԽ��Խ�� ��ʼʱm����yҲ��������m>2ʱ��m����y��С����m��2ʱ��y���������ӦΪm��2������0.45��
�����������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
��763K��3.04��104 kPaʱ����CO��H2Ϊԭ�Ϻϳ�CH3OH����������ƽ�⣺CO(g)+2H2(g) 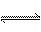 CH3OH(g)����ԭ����H2��CO�ı�����ͬʱ����CO��ת���ʼ�ƽ�������м״��������������Ӱ�졣
CH3OH(g)����ԭ����H2��CO�ı�����ͬʱ����CO��ת���ʼ�ƽ�������м״��������������Ӱ�졣
(1)��H2��CO����ʼ���ʵ�����Ϊm��ƽ��ʱCO��ת����Ϊa��ƽ�������м״����������Ϊy�����ƶ�y��a��m���ߵ����ϵ������y=f(a��m)�ĺ�������ʽ��ʾ������
(2)��������֪���ݴ��뵼���ı���ʽ�У��Ѽ���������ո��С�
m | a | y |
1 | 0.25 |
|
2 | 0.45 |
|
3 |
| 19.35% |
(3)���ݱ������ݣ��ж���CO��H2�ϳɼ״�ʱ��H2��CO�����������Ƕ��٣����������ݡ�