��Ŀ����
ij��Ȼ��Ļ�ѧ��ɿ���ΪaNa2CO3��bNaHCO3��cH2O(a��b��cΪ������)��Ϊȷ������ɣ�ijͬѧ��������̽����
(1)���Է���
ȡ������Ȼ����Ʒ�����Թ��У��þƾ��Ƽ��ȣ����Թܿ���Һ�����ɣ���Һ����ʹ��ˮ����ͭ�������ܷ�˵����Ʒ�к��ᾧˮ���Լ������ɣ�______________
(2)��������
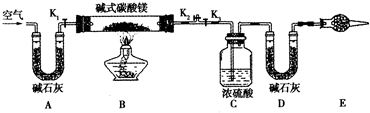
��ͬѧ�������ͼ��ʾװ�ã��ⶨ��Ȼ��Ļ�ѧ��ɣ�
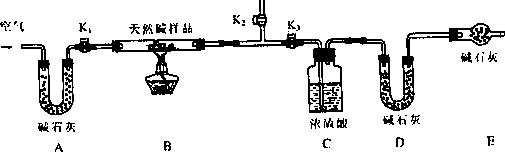
ʵ�鲽�裺
�ٰ���ͼ(�г�����δ����)��װ��ʵ��װ�ú����Ƚ��еIJ�����________��A����ʯ�ҵ������ǣ�_____________________��
�ڳ�ȡ��Ȼ����Ʒ7.3 g�����������Ӳ�ʲ������У�����װŨ�����ϴ��ƿ������Ϊ87.6 g��װ��ʯ�ҵ�U�ι�D������Ϊ74.7 g��
�۴���K1��K2���ر�K3���������˿��������ӣ�
�ܹرջ���Kl��K2����K3����ȼ�ƾ��Ƽ��ȣ������ٲ�������Ϊֹ��
�ݴ���Kl���������˿��������ӣ�Ȼ��Ƶ�װŨ�����ϴ��ƿ������Ϊ88.5 g��װ��ʯ�ҵ�U�ι�D������Ϊ75.8 g���ò����й������ʱ������Ŀ����________�������Ƶ�������Ȼ��Ļ�ѧʽΪ____________________��
(3)���ۣ��е�ͬѧ��Ϊ��Eװ���ǿ���ʡ�Եģ���Ĺ۵���________(���ܡ����ܡ�)�����ǣ�___________________________��
������
����(14��)(1)����˵������Ϊ��Ȼ����Ʒ�еġ�NaHCO3�����ȷֽ�Ҳ�ɲ���ˮ��(2��)
����(2)�ټ��װ�õ������ԡ���ȥͨ������е�CO2��H2O(4�֣�ÿ��2��)
������ʹ��Ӧ���ɵ�CO2��H2O���������(��ֹ�����ٶȹ��죬������̼��ˮ����δ����ȫ����)(2��)
���������Ƶ���Na2CO3��2NaHCO3��H2O(3��)
����(3)�����ܡ�(1��)�����еĶ�����̼��ˮ����������������˵�D�У�Ӱ�������̼�IJⶨ��(2��)