��Ŀ����
��1�������£���֪0.1mol?L-1һԪ��HA��Һ��c��OH-��/c��H+��=1×10-8���ٳ����£�0.1mol?L-1 HA��Һ��pH=______��д�����ᣨHA����NaOH��Һ��Ӧ�����ӷ���ʽ��______��
��pH=3��HA��pH=11��NaOH��Һ�������Ϻ���Һ��4���������ʵ���Ũ�ȴ�С��ϵ�ǣ�______��
��2�������£���pH=a�İ�ˮ�м�����������ʱ����Һ�����ԣ���������pH______14-a����������=��
��3�������ʵ���Ũ�Ⱦ�Ϊ0.01mol?L-1��MnCl2��BaCl2�����Һ�У��μ�Na2CO3��Һ���ȳ�����������______�����������ܵ���ʹ���ʱ����Һ��c��Ba2+��/c��Mn2+��______�������¶��£�Ksp��BaCO3��=8.1×10-9��Ksp��MnCO3��=1.8×10-11��
��4����Ka��Kh��Kw�ֱ��ʾCH3COOH�ĵ���ƽ�ⳣ����CH3COO-��ˮ��ƽ�ⳣ����ˮ�����ӻ�������������֮��Ĺ�ϵΪ��______��
���𰸡���������1���ٸ���c��OH-��/c��H+��=1×10-8���ˮ�����ӻ�������������������Ũ�ȣ��ٸ���ˮ�����ӻ�����������Һ��������Ũ�ȣ��Ӷ��ó���Һ��pH���������ӷ���ʽ��д������д���ӷ���ʽ��
�ڸ�����ͼ�����ʵ�������Դ�Сȷ����Һ�е����ʣ��Ӷ�ȷ����Һ������ԣ���ϵ���غ�ȷ����Һ�и�������Ũ�ȵ���Դ�С��
��2�����ü��跨�ж������pHֵ��ʵ���������pHֵС�ڼ���ֵ��
��3����������ܶȻ�����ԽС�ģ��ý��������ȳ��������������ܵ���ʹ���ʱ��̼����ǡ�ñ��ͣ���Һ��������Ũ�Ⱥ�̼�������Ũ����ȣ�����̼���̵��ܶȻ��������������ӡ�̼�������Ũ�ȣ��ٸ���̼�ᱵ�ܶȻ�������̼�������Ũ�ȼ��㱵����Ũ�ȣ��Ӷ��ó������Ӻ�������Ũ��֮�ȣ�
��4��д���������ƽ�ⳣ�������������ˮ��ƽ�ⳣ����ˮ�����ӻ��������бȽϣ��Ӷ��ó����ۣ�
����⣺��1����c��OH-��/c��H+��=1×10-8��c��OH-��×c��H+��=1×10-14������c��OH-��=10-11mol?L-1����c��H+��=10-3mol?L-1��������Һ��pH=3��������Ũ��С�����Ũ�ȣ����Ը��������ᣬ���ᣨHA����NaOH��Һ��Ӧ�����ӷ���ʽΪ��HA+OH-�TA-+H2O��
�ʴ�Ϊ��3��HA+OH-�TA-+H2O��
��pH=11��NaOH��Һ��c��OH-��=10-3mol?L-1��HA�����ᣬ���Ũ��ԶԶ����������Ũ�ȣ�����pH=3��HA��pH=11��NaOH��Һ�������Ϻ���Һ�е�����������Σ���Һ�����ԣ�������Һ��������Ũ�ȴ�������������Ũ�ȣ��������Ũ�ȴ���������Ũ�ȣ���Һ��4���������ʵ���Ũ�ȴ�С��ϵ��c��A-����c��Na+����c��H+����c��OH-����
�ʴ�Ϊ��c��A-����c��Na+����c��H+����c��OH-����
��2�����谱ˮ��ǿ�����ʱ��pH=a�İ�ˮ������������Ũ��=10a-14mol/L����pH=a�İ�ˮ�м�����������ʱ����Һ�����ԣ�������������Ũ�ȵ���������Ũ�ȣ����������pH=14-a��ʵ���ϰ�ˮ��������������ͼ��Ϻ���Һ�����ԣ�˵�����Ũ�ȴ��ڼ��Ũ�ȣ��������pH��14-a����ѡ����
��3��̼�ᱵ���ܶȻ���������̼���̵��ܶȻ������������������ȳ��������������ܵ���ʹ���ʱ����
c��CO32-��=c��Mn2+��=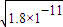 ����Һ��c��Ba2+��=
����Һ��c��Ba2+��=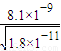 ��c��Ba2+����c��Mn2+��=
��c��Ba2+����c��Mn2+��=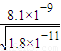 ��
��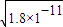 =450��
=450��
�ʴ�Ϊ��Mn2+��450��
��4��Ka=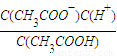 ��Kh=
��Kh=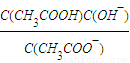 ��Kw=C��H+����C��OH-��������Ka?Kh=Kw��
��Kw=C��H+����C��OH-��������Ka?Kh=Kw��
�ʴ�Ϊ��Ka?Kh=Kw��
���������⿼��������Ũ�ȴ�С�ıȽϡ��ܶȻ����йؼ����֪ʶ�㣬�ѶȽϴ�ע�����ü��跨���жԣ�2������м���ϼ�㣬Ϊ�״��⣮
�ڸ�����ͼ�����ʵ�������Դ�Сȷ����Һ�е����ʣ��Ӷ�ȷ����Һ������ԣ���ϵ���غ�ȷ����Һ�и�������Ũ�ȵ���Դ�С��
��2�����ü��跨�ж������pHֵ��ʵ���������pHֵС�ڼ���ֵ��
��3����������ܶȻ�����ԽС�ģ��ý��������ȳ��������������ܵ���ʹ���ʱ��̼����ǡ�ñ��ͣ���Һ��������Ũ�Ⱥ�̼�������Ũ����ȣ�����̼���̵��ܶȻ��������������ӡ�̼�������Ũ�ȣ��ٸ���̼�ᱵ�ܶȻ�������̼�������Ũ�ȼ��㱵����Ũ�ȣ��Ӷ��ó������Ӻ�������Ũ��֮�ȣ�
��4��д���������ƽ�ⳣ�������������ˮ��ƽ�ⳣ����ˮ�����ӻ��������бȽϣ��Ӷ��ó����ۣ�
����⣺��1����c��OH-��/c��H+��=1×10-8��c��OH-��×c��H+��=1×10-14������c��OH-��=10-11mol?L-1����c��H+��=10-3mol?L-1��������Һ��pH=3��������Ũ��С�����Ũ�ȣ����Ը��������ᣬ���ᣨHA����NaOH��Һ��Ӧ�����ӷ���ʽΪ��HA+OH-�TA-+H2O��
�ʴ�Ϊ��3��HA+OH-�TA-+H2O��
��pH=11��NaOH��Һ��c��OH-��=10-3mol?L-1��HA�����ᣬ���Ũ��ԶԶ����������Ũ�ȣ�����pH=3��HA��pH=11��NaOH��Һ�������Ϻ���Һ�е�����������Σ���Һ�����ԣ�������Һ��������Ũ�ȴ�������������Ũ�ȣ��������Ũ�ȴ���������Ũ�ȣ���Һ��4���������ʵ���Ũ�ȴ�С��ϵ��c��A-����c��Na+����c��H+����c��OH-����
�ʴ�Ϊ��c��A-����c��Na+����c��H+����c��OH-����
��2�����谱ˮ��ǿ�����ʱ��pH=a�İ�ˮ������������Ũ��=10a-14mol/L����pH=a�İ�ˮ�м�����������ʱ����Һ�����ԣ�������������Ũ�ȵ���������Ũ�ȣ����������pH=14-a��ʵ���ϰ�ˮ��������������ͼ��Ϻ���Һ�����ԣ�˵�����Ũ�ȴ��ڼ��Ũ�ȣ��������pH��14-a����ѡ����
��3��̼�ᱵ���ܶȻ���������̼���̵��ܶȻ������������������ȳ��������������ܵ���ʹ���ʱ����
c��CO32-��=c��Mn2+��=
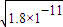 ����Һ��c��Ba2+��=
����Һ��c��Ba2+��=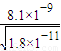 ��c��Ba2+����c��Mn2+��=
��c��Ba2+����c��Mn2+��=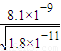 ��
��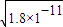 =450��
=450���ʴ�Ϊ��Mn2+��450��
��4��Ka=
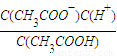 ��Kh=
��Kh=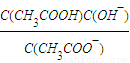 ��Kw=C��H+����C��OH-��������Ka?Kh=Kw��
��Kw=C��H+����C��OH-��������Ka?Kh=Kw���ʴ�Ϊ��Ka?Kh=Kw��
���������⿼��������Ũ�ȴ�С�ıȽϡ��ܶȻ����йؼ����֪ʶ�㣬�ѶȽϴ�ע�����ü��跨���жԣ�2������м���ϼ�㣬Ϊ�״��⣮

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 I���±���ʵ�����Ʊ�������й����ݣ�
I���±���ʵ�����Ʊ�������й����ݣ�
| ��� | ʵ������ | ʵ��ԭ�� | ����װ�� |
| �� | ������ | H2O2��O2 | |
| �� | �ư��� | NH4Cl��NH3 | |
| �� | ������ | HCl��Cl2 |
��2�����ݱ�������ʵ��ԭ����������װ����ѡ����ʵķ���װ�ã������������ϱ��Ŀո��У�����
��3�����������Ʊ�O2��װ���Ʊ�NH3����ѡ����Լ�Ϊ______��
��֪��NH3?H2O�ĵ��뷽��ʽΪ��NH3?H2O?NH+4+OH-����д��NH3����ˮ���γɵ�NH3?H2O�ĺ����ṹ______
��4���Ʊ�Cl2����8mol?L-1������100mL������12mol?L-1�����������ƣ�
����Ҫ12mol?L-1����������Ϊ______mL����ȷ��0.1mL��
��������ƿ��ʹ�÷����У����в�������ȷ����______����д��ţ���
A��ʹ������ƿǰ������Ƿ�©ˮ
B������ƿ������ˮϴ�������ô�����Һ��ϴ
C��������Һʱ����Ͳ��ȡŨ������ò���������������ƿ�У�������������ˮ���ӽ�����1cm��2cm�����ý�ͷ�ιܵμ�����ˮֱ����Һ�����ʹ��ͱ�����ƽ
D�����ݺ�Ǻ�ƿ������ʳָ��ס������һֻ����ָ��סƿ�ף�������ƿ��ת��ҡ�����
II����1�������£���֪0.1mol?L-1һԪ��HA��Һ��c��OH-��/c��H+��=1��10-8��
д������HA��NaOH��Һ��Ӧ�����ӷ���ʽʽ��______��
��2��t��ʱ����pH=2��ϡ�����pH=11��NaOH��Һ�������Ϻ���Һ�����ԣ�
����¶��£�t�棩����100mL 0.1mol?L-1��ϡH2SO4��Һ��100mL 0.4mol?L-1��NaOH��Һ��Ϻ���Һ����仯���Բ��ƣ�����Һ��pH=______��
 I���±���ʵ�����Ʊ�������й����ݣ�
I���±���ʵ�����Ʊ�������й����ݣ�