��Ŀ����

(2)V��W��Z����Ԫ�ص����Ӿ�����ͬ�ĵ��Ӳ�ṹ�����ߵ����Ӱ뾶�ɴ�С˳����____���������ӷ��ű�ʾ���������ӷ���ʽ��ʾ��Z���ӿ�����ˮ����ԭ��____��
(3)ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ��____��
(4)1 g YU4������ȫȼ������Һ̬ˮʱ���ų�akJ����������÷�Ӧ���Ȼ�ѧ����ʽΪ________��
(5)����Z��V2W2�Ĺ���������Ʒ������ϡ�������������ȫ�ܽ⣬���õĻ�� Һ��c(Z3+ )��c(H+)��c(Cl-)=1��2��8����ԭ���������У�ZԪ����WԪ�ص�������Ϊ____����������ȣ���
(2)O2- >Na+>Al3+ ��Al3+ +3H2O
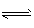 Al(OH)3�����壩+3 H+
Al(OH)3�����壩+3 H+ (3)
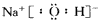 (Na2O2Ҳ����)
(Na2O2Ҳ����) (4)CH4(g)+2O2(g)==CO2(g)+H2O(l)��H=-16akJ/mol
(5)9 : 16

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���12�֣��±��Ƕ������в���Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼۡ�
| Ԫ�ش��� | W | R | X | Y | Z |
| ԭ�Ӱ뾶/nm | 0��037 | 0��157 | 0��066 | 0��070 | 0��077 |
| ��Ҫ���ϼ� | +1 | ��1 | -2 | -3��+5 | +2��+4 |
��ش�
��1��Z��Ԫ�����ڱ��е�λ���� ��Z������Y������������Ӧˮ�����Ũ��Һ��Ӧ������һ�ֺ���ɫ���壬��Ӧ�Ļ�ѧ����ʽΪ ��
��2��ʵ������ȡYW3�Ļ�ѧ����ʽΪ ��
��3��R2X2��W2X��Ӧ�����ӷ���ʽΪ ������Ӧ��ת�Ƶĵ�����Ϊ0.4��6.02��1023����μӷ�Ӧ��R2X2������Ϊ ��
��4��X��Z���һ���г�ζ�����壬�����������ʵ���֮��Ϊ1:2��Ϻ�ǡ����ȫȼ�գ������ȶ����������ͬ��ͬѹ�²��ȼ��ǰ���������������䣬��÷�Ӧ�Ļ�ѧ����ʽ�� ��
��12�֣��±��Ƕ������в���Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼۡ�
| Ԫ�ش��� | W | R | X | Y | Z |
| ԭ�Ӱ뾶/nm | 0��037 | 0��157 | 0��066 | 0��070 | 0��077 |
| ��Ҫ���ϼ� | +1 | ��1 | -2 | -3��+5 | +2��+4 |
��1��Z��Ԫ�����ڱ��е�λ���� ��Z������Y������������Ӧˮ�����Ũ��Һ��Ӧ������һ�ֺ���ɫ���壬��Ӧ�Ļ�ѧ����ʽΪ ��
��2��ʵ������ȡYW3�Ļ�ѧ����ʽΪ ��
��3��R2X2��W2X��Ӧ�����ӷ���ʽΪ ������Ӧ��ת�Ƶĵ�����Ϊ0.4��6.02��1023����μӷ�Ӧ��R2X2������Ϊ ��
��4��X��Z���һ���г�ζ�����壬�����������ʵ���֮��Ϊ1:2��Ϻ�ǡ����ȫȼ�գ������ȶ����������ͬ��ͬѹ�²��ȼ��ǰ���������������䣬��÷�Ӧ�Ļ�ѧ����ʽ�� ��
��12�֣��±��Ƕ������в���Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼۡ�
|
Ԫ�ش��� |
W |
R |
X |
Y |
Z |
|
ԭ�Ӱ뾶/nm |
0��037 |
0��157 |
0��066 |
0��070 |
0��077 |
|
��Ҫ���ϼ� |
+1 |
��1 |
-2 |
-3��+5 |
+2��+4 |
��ش�
��1��Z��Ԫ�����ڱ��е�λ���� ��Z������Y������������Ӧˮ�����Ũ��Һ��Ӧ������һ�ֺ���ɫ���壬��Ӧ�Ļ�ѧ����ʽΪ ��
��2��ʵ������ȡYW3�Ļ�ѧ����ʽΪ ��
��3��R2X2��W2X��Ӧ�����ӷ���ʽΪ ������Ӧ��ת�Ƶĵ�����Ϊ0.4��6.02��1023����μӷ�Ӧ��R2X2������Ϊ ��
��4��X��Z���һ���г�ζ�����壬�����������ʵ���֮��Ϊ1:2��Ϻ�ǡ����ȫȼ�գ������ȶ����������ͬ��ͬѹ�²��ȼ��ǰ���������������䣬��÷�Ӧ�Ļ�ѧ����ʽ�� ��
(08�㽭ʡ������ʮ��У�¿�)�±��Ƕ������в���Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼۡ�
Ԫ�ش��� | U | V | W | X | Y | Z |
ԭ�Ӱ뾶/nm | 0.037 | 0.157 | 0.066 | 0.070 | 0.077 | 0.143 |
��Ҫ���ϼ� | +1 | +1 | ��2 | ��3,+5 | +2,+4 | +3 |
��ش�
��1��Y��Ԫ�����ڱ��е�λ���� ��
��2��V��W��Z����Ԫ�ص����Ӿ�����ͬ�ĵ��Ӳ�ṹ�����ߵ����Ӱ뾶�ɴ�С˳����
����Ԫ�ط��ű�ʾ����
�������ӷ���ʽ��ʾ��Z���ӿ�����ˮ����ԭ��
��3��W��X��Y�ֱ���U���γ�10���ӹ��ۻ�������߾����γ� ���壬���зе���ߵ��ǣ�д��ѧʽ��__ ��1g YU4������ȫȼ������Һ̬ˮʱ���ų�a kJ������,��÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��4������Z��V2W2�Ĺ���������Ʒ������ϡ�������������ȫ�ܽ⣬���õĻ��Һ��c(Z3��)c(H��)c(Cl��)��128����ԭ���������У�ZԪ����WԪ�ص�������Ϊ ����������ȣ���