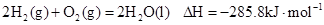��Ŀ����
��Ҫ��������и�С�⣺
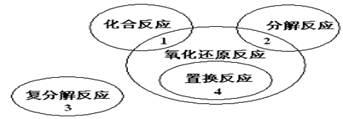
��1���ҹ��Ŵ��Ĵ���֮һ�ĺڻ�ҩ����ըʱ�ķ�ӦΪS+2KNO3+3C=K2S+N2��+3CO2���÷�Ӧ�л�ԭ���� ���������� ��
��2������ͨ������ԭ���� ������ ����ˮ�����ͨ�����Ĺ�ͬԭ�ϡ�
��3�������������ʽ��ʾij�����ε���ɣ�K4Al4FeSi6O18(OH)7 .
��4��Ư�۵ijɷ��� ����ҵ��Ư�۵Ļ�ѧ����ʽΪ Ư���ڿ����б��治����ʧЧ��ԭ���û�ѧ����ʽ��ʾ
��5����23gNa��28g��ͬʱͶ��ˮ�У������Ϸų�H2 g��д����Ӧ���йص����ӷ���ʽ ��
��1���ҹ��Ŵ��Ĵ���֮һ�ĺڻ�ҩ����ըʱ�ķ�ӦΪS+2KNO3+3C=K2S+N2��+3CO2���÷�Ӧ�л�ԭ���� ���������� ��
��2������ͨ������ԭ���� ������ ����ˮ�����ͨ�����Ĺ�ͬԭ�ϡ�
��3�������������ʽ��ʾij�����ε���ɣ�K4Al4FeSi6O18(OH)7 .
��4��Ư�۵ijɷ��� ����ҵ��Ư�۵Ļ�ѧ����ʽΪ Ư���ڿ����б��治����ʧЧ��ԭ���û�ѧ����ʽ��ʾ
��5����23gNa��28g��ͬʱͶ��ˮ�У������Ϸų�H2 g��д����Ӧ���йص����ӷ���ʽ ��
��1��C S��KNO3
��2�����ʯ��ʯ��ʯӢ ʯ��ʯ
��3��4K2O��4Al2O3��Fe2O3��12SiO2��7H2O
��4��CaCl2��Ca(ClO)2 2Cl2+2Ca(OH)2=CaCl2+Ca(ClO)2+2H2O
Ca(ClO)2+CO2+H2O=CaCO3+2HClO (δд��2HClO 2HCl��O2�����۷�)
2HCl��O2�����۷�)
��5��3g 2Na+2H2O=2Na++2OH-+H2 Si+2OH-+H2O=SiO32-+2H2
��2�����ʯ��ʯ��ʯӢ ʯ��ʯ
��3��4K2O��4Al2O3��Fe2O3��12SiO2��7H2O
��4��CaCl2��Ca(ClO)2 2Cl2+2Ca(OH)2=CaCl2+Ca(ClO)2+2H2O
Ca(ClO)2+CO2+H2O=CaCO3+2HClO (δд��2HClO
 2HCl��O2�����۷�)
2HCl��O2�����۷�)��5��3g 2Na+2H2O=2Na++2OH-+H2 Si+2OH-+H2O=SiO32-+2H2
��1����Ӧ��̼�Ļ��ϼ���0������+4�ۣ�������������ԭ����������0�۽�����2�ۡ�����+5�۽���0�ۣ���S��KNO3����������
��2������ͨ������ԭ���Ǵ��ʯ��ʯ��ʯӢ����ˮ���ԭ��Ϊʯ��ʯ��ճ��
��3����ԭ���غ��֪��������ʽΪ��4K2O��4Al2O3��Fe2O3��12SiO2��7H2O
��4����ҵ�Ͻ�����ͨ��ʯ��������ȡƯ�ۣ�2Cl2+2Ca(OH)2=CaCl2+Ca(ClO)2+2H2O��Ư�۵���Ҫ�ɷ�ΪCaCl2��Ca(ClO)2�Ļ����������е�Ca(ClO)2�����տ����е�CO2��ʹ��ʧЧ��Ca(ClO)2+CO2+H2O=CaCO3+2HClO��2HClO 2HCl��O2������Ư����Ҫ�ܷⱣ��
2HCl��O2������Ư����Ҫ�ܷⱣ��
��5��������������Ӧ֪��

������ȫ����ˮ��Ӧ�����ɵļ���Թ��������㣬�������ɵ�������1.5mol������Ϊ3g
��2������ͨ������ԭ���Ǵ��ʯ��ʯ��ʯӢ����ˮ���ԭ��Ϊʯ��ʯ��ճ��
��3����ԭ���غ��֪��������ʽΪ��4K2O��4Al2O3��Fe2O3��12SiO2��7H2O
��4����ҵ�Ͻ�����ͨ��ʯ��������ȡƯ�ۣ�2Cl2+2Ca(OH)2=CaCl2+Ca(ClO)2+2H2O��Ư�۵���Ҫ�ɷ�ΪCaCl2��Ca(ClO)2�Ļ����������е�Ca(ClO)2�����տ����е�CO2��ʹ��ʧЧ��Ca(ClO)2+CO2+H2O=CaCO3+2HClO��2HClO
 2HCl��O2������Ư����Ҫ�ܷⱣ��
2HCl��O2������Ư����Ҫ�ܷⱣ����5��������������Ӧ֪��

������ȫ����ˮ��Ӧ�����ɵļ���Թ��������㣬�������ɵ�������1.5mol������Ϊ3g

��ϰ��ϵ�д�
�����Ŀ
 NaCl
NaCl
 �Ľṹʾ��ͼ:
�Ľṹʾ��ͼ:

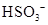 ���ӵĵ��뷽��ʽ:
���ӵĵ��뷽��ʽ: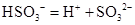
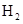 ��25�� 101kpa��ȼ�գ�����Һ̬��ˮ�ų�285.8
��25�� 101kpa��ȼ�գ�����Һ̬��ˮ�ų�285.8 U����������÷�Ӧ���Ȼ�ѧ����ʽΪ:
U����������÷�Ӧ���Ȼ�ѧ����ʽΪ: