题目内容
【题目】I.写出下列反应的化学方程式,并指出反应类型:
(1)用乙烯制备聚乙烯:________________________________;反应类型_________。
(2)乙醇在加热有铜作催化剂的条件下反应:______________________________;反应类型__________。
(3)乙酸乙酯在稀硫酸加热条件下水解:_________________________________________;反应类型__________。
II.现有下列五种有机物:①CH4、②CH2=CH2、③CH3CH2OH、④ CH3 CH2CH2 CH3 、⑤CH3COOH。请回答:
(1)写出⑤的官能团的名称______。
(2)与①互为同系物的是______(填序号)。
(3)写出④ 的同分异构体的结构简式________________。
【答案】 nCH2=CH2![]()
![]() 加聚反应 2CH3CH2OH+O2
加聚反应 2CH3CH2OH+O2![]() 2CH3CHO+2H2O 氧化反应 CH3COOCH2CH3+H2O
2CH3CHO+2H2O 氧化反应 CH3COOCH2CH3+H2O![]() CH3COOH+ CH3CH2OH 取代反应 羧基 ④ CH3 CH (CH3 )CH3
CH3COOH+ CH3CH2OH 取代反应 羧基 ④ CH3 CH (CH3 )CH3
【解析】I.(1)乙烯发生加成聚合反应可以制备聚乙烯,反应为:nCH2=CH2![]()
![]() ,属于加聚反应;(2)乙醇在加热有铜作催化剂的条件下反应生成乙醛和水,反应方程式为:2CH3CH2OH+O2
,属于加聚反应;(2)乙醇在加热有铜作催化剂的条件下反应生成乙醛和水,反应方程式为:2CH3CH2OH+O2![]() 2CH3CHO+2H2O;反应类型氧化反应;(3)乙酸乙酯在稀硫酸加热条件下水解生成乙酸和乙醇,反应方程式为:CH3COOCH2CH3+H2O
2CH3CHO+2H2O;反应类型氧化反应;(3)乙酸乙酯在稀硫酸加热条件下水解生成乙酸和乙醇,反应方程式为:CH3COOCH2CH3+H2O![]() CH3COOH+ CH3CH2OH;反应类型取代反应;II.(1)⑤为CH3COOH,属于羧酸,其官能团的名称为羧基;(2)与①(甲烷属于烷烃)互为同系物的是④CH3CH2CH2CH3(丁烷也属于烷烃);(3)④ 的同分异构体有异丁烷,其结构简式为CH3CH(CH3)CH3。
CH3COOH+ CH3CH2OH;反应类型取代反应;II.(1)⑤为CH3COOH,属于羧酸,其官能团的名称为羧基;(2)与①(甲烷属于烷烃)互为同系物的是④CH3CH2CH2CH3(丁烷也属于烷烃);(3)④ 的同分异构体有异丁烷,其结构简式为CH3CH(CH3)CH3。

【题目】某化学兴趣小组设计实验,用浓硫酸与铜的反应制取SO2并进行相关实验探究,同时获得少量NaHSO3,实验装置如下图所示:
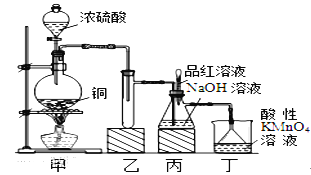
(1)装置甲中发生反应的化学方程式是________________,装置乙的作用是___________。
(2)装置丁的作用是吸收污染空气的SO2气体,其反应的离子方程为___________________。
(3)SO2气体有漂白性、还原性和氧化性。将SO2通入溴水中,SO2表现的是__________性,化学反应方程式为_________________________________。
(4)反应过程中,将丙中滴管里的品红溶液滴入锥形瓶,若现象为_____________,则溶液中的NaOH完全转化为了NaHSO3。
(5)若丙中没有加入品红溶液,则不能准确判断氢氧化钠是否完全转化。现有可供选择的仪器和试剂:烧杯、试管、玻璃棒、胶头滴管; 2mol/L盐酸、2mol/L硝酸、1mol/L氯化钡溶液、1 mol/L氢氧化钡溶液、品红溶液、蒸馏水。请设计实验探究吸收后产物中是否存在NaHSO3和Na2SO3,将实验操作、预期的实验现象和结论填在下表中。
实验操作 | 预期现象与结论 |
步骤1:取少量待测液放入试管中,滴加过量1 mol/L氯化钡溶液。静置一段时间后,得到滤液A和固体B。 | (空) |
步骤2:往固体B中加入蒸馏水洗涤沉淀,静置后弃去上层清液,向固体滴入2滴(或少量)品红,再滴加__________________。 | 若品红褪色(或有气泡),则说明含有Na2SO3。 |
步骤3:用试管取少量A,向其中加入过量___________。 | 若生成白色沉淀,则说明有NaHSO3生成 |