��Ŀ����
��17�֣�ij�л���A����C��H��O����Ԫ����ɣ���һ�������£���A����ת��Ϊ�л���B��C��D��E��ת���ϵ���£�
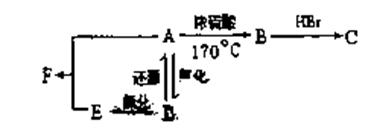
��֪D����Է�������Ϊ44�����ɷ���������Ӧ��
��1��д��D�����ƣ�
 ��2��������C�Ľṹ��ʽΪ
��2��������C�Ľṹ��ʽΪ
��3��д��E��ͬ���칹��Ľṹ��ʽ
��4��д��ʵ������ת���Ļ�ѧ����ʽ��ע�������ͷ�Ӧ���ͣ��л���д�ṹ��ʽ����
A��B�� ����Ӧ���� ��
A��D�� ����Ӧ���� ��
A��E��F��H2O�� ����Ӧ���� ��

��֪D����Է�������Ϊ44�����ɷ���������Ӧ��
��1��д��D�����ƣ�
 ��2��������C�Ľṹ��ʽΪ
��2��������C�Ľṹ��ʽΪ ��3��д��E��ͬ���칹��Ľṹ��ʽ
��4��д��ʵ������ת���Ļ�ѧ����ʽ��ע�������ͷ�Ӧ���ͣ��л���д�ṹ��ʽ����
A��B�� ����Ӧ���� ��
A��D�� ����Ӧ���� ��
A��E��F��H2O�� ����Ӧ���� ��
��1����ȩ��1�֣���2��CH3C H2Br��2�֣���3��HCOOCH3��2�֣�
H2Br��2�֣���3��HCOOCH3��2�֣�
��3��CH3CH2OH CH2=CH2����H2O ��ȥ��Ӧ
CH2=CH2����H2O ��ȥ��Ӧ
2CH3CH2OH��O2 2CH3CHO��2H2O ������Ӧ
2CH3CHO��2H2O ������Ӧ
CH3CH2OH ��CH3COOH
��CH3COOH CH3COOC2H5��H2O ������Ӧ��ȡ����Ӧ
CH3COOC2H5��H2O ������Ӧ��ȡ����Ӧ
������ʽÿ��3�֣���Ӧ����1�ˣ�������д������ʽ��ȷֻ��1�֣�
 H2Br��2�֣���3��HCOOCH3��2�֣�
H2Br��2�֣���3��HCOOCH3��2�֣���3��CH3CH2OH
 CH2=CH2����H2O ��ȥ��Ӧ
CH2=CH2����H2O ��ȥ��Ӧ2CH3CH2OH��O2
 2CH3CHO��2H2O ������Ӧ
2CH3CHO��2H2O ������ӦCH3CH2OH
 ��CH3COOH
��CH3COOH CH3COOC2H5��H2O ������Ӧ��ȡ����Ӧ
CH3COOC2H5��H2O ������Ӧ��ȡ����Ӧ������ʽÿ��3�֣���Ӧ����1�ˣ�������д������ʽ��ȷֻ��1�֣�
��

��ϰ��ϵ�д�
�����Ŀ
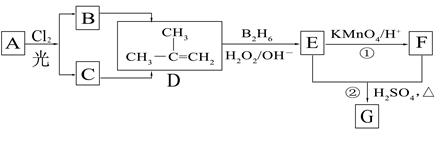
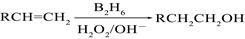 ��B2H6Ϊ�����飩
��B2H6Ϊ�����飩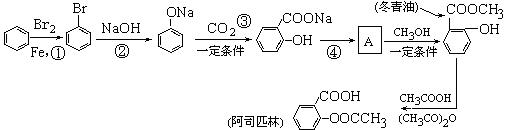
 ��1��д������ת���ڵĻ�ѧ��Ӧ����ʽ��
��1��д������ת���ڵĻ�ѧ��Ӧ����ʽ�� ___________________________________________��
___________________________________________��
 CH3OH(g)��H2O(g) ����H����49.0kJ/mol�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
CH3OH(g)��H2O(g) ����H����49.0kJ/mol�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��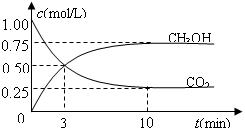
 ����CO����Ⱦ��������
����CO����Ⱦ�������� ���Ƿ���в�˵�����ɣ�����������������������������������������������������������������
���Ƿ���в�˵�����ɣ�����������������������������������������������������������������
 ��ͬѹ��4.75����������Իش�
��ͬѹ��4.75����������Իش� ��Ľṹ��ʽΪ ��
��Ľṹ��ʽΪ �� ��˵���У���ȷ����
��˵���У���ȷ����