��Ŀ����
����Ŀ����6 g CaCO3��100 mLϡ���ᷴӦ��ȡ������CO2����Ӧ���������ɵ�CO2�������������Ϊ��״�����淴Ӧʱ��仯���������ͼ��ʾ������˵����ȷ������ ��
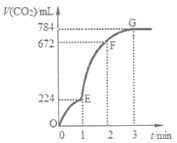
A��OE�α�ʾ��ƽ����Ӧ������죬���ܵ�ԭ���Ǹ÷�Ӧ�Ƿ��ȷ�Ӧ
B��EF����HC1Ũ�ȵļ�С��ʾ�ĸ÷�Ӧ��ƽ����Ӧ����Ϊ0.2 mol/��L��min��
C����F���ռ�����CO2��������
D����G���Ժ��ռ���CO2�����������࣬ԭ����ϡ�����ѷ�Ӧ��ȫ
���𰸡�D
��������
���������A����Һ��������Ũ��Խ��Ӧ����Խ�죬Խ��Ӧ�÷�Ӧ����Խ�죬�÷�Ӧ�Ƿ��ȷ�Ӧ�����տ�ʼʱ��Ũ�ȵ�Ӱ������¶ȵ�Ӱ�죬��Ӧ���������ߵ����߳����ȣ�����ͼ֪OE�α�ʾ��ƽ����Ӧ������죬ԭ����ϡ����Ũ�ȴ���B����ͼ��֪EF�����ɵĶ�����̼���Ϊ672mL-224mL=448mL��n��CO2��=0.448L��22.4L/mol=0.02mol�����ݶ�����̼��ϡ����Ĺ�ϵʽ�òμӷ�Ӧ��n��HCl��=2 n��CO2��=0.02mol��2=0.04mol����HCl��ƽ����Ӧ����v��HCl��=0.04mol��0.1L��1min=0.4 mol/��Lmin��������C�������ϵ���������ֵ��Ϊ�õ��ռ��Ķ�����̼�������������G���ռ�����CO2����������D��������̼������Ϊ784mL��n��CO2��=0.784L��22.4L/mol=0.035mol������Cԭ���غ�֪�μӷ�Ӧ��n��CaCO3��=0.035mol��6g��100g/mol=0.06mol��˵��̼�����ʣ�࣬��ϡ������ȫ�����ģ���ȷ��

 ȫ��������ϵ�д�
ȫ��������ϵ�д�