ΧβΡΩΡΎ»ί
‘Ύ120ΓφΓΔ101 kPaΧθΦΰœ¬Θ§”…H2ΓΔCH4ΓΔCOΉι≥…ΒΡΜλΚœΤχΧεa mLΘ§Ά®»κ“ΜΕ®ΝΩ(…ηΈΣx mL)―θΤχ ΙΤδΆξ»Ϊ»Φ…’ΓΘ
(1)»τa mLΜλΚœΤχΧεΆξ»Ϊ»Φ…’œϊΚΡœύΆ§ΧθΦΰœ¬―θΤχΒΡΧεΜΐ“≤ΈΣa mLΘ§(Φ¥xΘΫa)Θ§‘ρ‘≠ΜλΚœΤχΧε÷–CH4ΒΡΧεΜΐΖ÷ ΐ « ΘΜ
(2)»τΆξ»Ϊ»Φ…’Κσ…ζ≥…CO2ΚΆH2O(g)ΒΡΉήΧεΜΐ‘ΎœύΆ§ΧθΦΰœ¬ΈΣ2a mLΘ§‘ρ‘≠ΜλΚœΤχΧε÷–CH4ΒΡΧεΜΐΖ÷ ΐ « Θ§œ÷“Σ≤βΕ®‘≠ΜλΚœΤχΧε÷–H2ΒΡΧεΜΐΖ÷ ΐΘ§ΜΙ±Ί–κ÷ΣΒάœύΆ§ΧθΦΰœ¬ΒΡΤδΥϊ ΐΨίΘ§Ω…“‘ « (Χν―ΓœνΉ÷ΡΗ)ΘΜ
AΘ°2a mLΜλΚœΤχΧεΒΡΟήΕ»
BΘ°…ζ≥…CO2ΤχΧεΒΡΉήΧεΜΐ
CΘ°…ζ≥…H2O(g)ΒΡΉή÷ ΝΩ
(3)»τ‘≠ΜλΚœΤχΧεΆξ»Ϊ»Φ…’ ±Θ§…ζ≥…ΒΡΤχΧε÷–÷Μ”–CO2ΚΆH2O(g)Θ§‘ρxΒΡ»Γ÷ΒΖΕΈß « ΓΘ
(1)33.3% (2)50% B (3)0.5aΘΦxΘΦ2a
ΓΨΫβΈωΓΩ(1)Β»ΧεΜΐH2ΓΔCO‘Ύ―θΤχ÷–Άξ»Ϊ»Φ…’ ±Θ§œϊΚΡΒ»ΧεΜΐΒΡ―θΤχΘ§…ηH2ΚΆCOΧεΜΐΚΆΈΣxΘ§CH4ΒΡΧεΜΐΈΣyΘ§‘ρxΘΪyΘΫ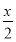 ΘΪ2yΘ§ΫβΒΟyΘΚxΘΫ1ΘΚ2Θ§Υυ“‘CH4ΒΡΧεΜΐΖ÷ ΐΈΣ33.3%ΓΘ
ΘΪ2yΘ§ΫβΒΟyΘΚxΘΫ1ΘΚ2Θ§Υυ“‘CH4ΒΡΧεΜΐΖ÷ ΐΈΣ33.3%ΓΘ
(2)Ά§άμΘ§H2ΚΆCO»Φ…’…ζ≥…H2OΚΆCO2ΧεΜΐΚΆΈΣxΘ§CH4»Φ…’…ζ≥…2yΧεΜΐΒΡH2OΚΆyΧεΜΐΒΡCO2Θ§Υυ“‘xΘΪ3yΘΫ2(xΘΪy)Θ§ΫβΒΟxΘΚyΘΫ1ΘΚ1Θ§Υυ“‘CH4ΒΡΧεΜΐΖ÷ ΐΈΣ50%ΓΘ
(3)»τa mL»Ϊ≤Ω «COΜρH2Θ§‘ρΆξ»Ϊ»Φ…’ ±œϊΚΡO2ΒΡΧεΜΐΈΣ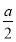 mLΘΜ»τa mL»Ϊ≤Ω «CH4Θ§‘ρΆξ»Ϊ»Φ…’ ±œϊΚΡO2ΒΡΧεΜΐΈΣ2a mLΘ§Υυ“‘ΒΟ0.5aΘΦxΘΦ2aΓΘ
mLΘΜ»τa mL»Ϊ≤Ω «CH4Θ§‘ρΆξ»Ϊ»Φ…’ ±œϊΚΡO2ΒΡΧεΜΐΈΣ2a mLΘ§Υυ“‘ΒΟ0.5aΘΦxΘΦ2aΓΘ