��Ŀ����
����Ŀ����ľ���и������Σ���Ҫ�ɷ���̼��أ������������Ȼ��غ�����صȡ��ִ�ij��ľ����Ʒ����ȡ���Σ����������е�![]() ��
��![]() ��Cl��
��Cl��
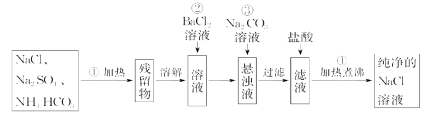
(1)�Ӳ�ľ������ȡ���ε�ʵ������������£��벹��������
�� ������ ������Ũ�� ����ȴ�ᾧ �����ˡ�
(2)������������Ҫ�õ����������� (�����)��
(3)���Ƶõ����������ˮ�ܽ�ֱ�������֧�Թ��С�
�����һ֧�Թ��м�������ϡ���ᣬ�ɹ۲쵽 ��˵����Һ�д��� ���ӡ�
����ڶ�֧�Թ��м��� ���ɹ۲쵽 ��˵����Һ�д���![]() ��
��
�������֧�Թ����ȼ�������Ba(NO3)2��Һ�����˺�������Һ�м������� ��Һ���ɹ۲쵽��ɫ������˵����Һ�д���Cl�����У���������Ba(NO3)2��Һ��Ŀ���� ��
���𰸡�(1)��ˮ����(���ܽ�)
(2)�٢ڢۢ�
(3)�������������� ![]()
������ϡ������ټ���BaCl2��Һ ��ɫ����
��(�����ữ��)AgNO3��Һ �ų�![]() ��
��![]() �ĸ���
�ĸ���
��������(1)��ľ���к��е����ӻ�����Ϊ��̬��������Ҫ����ľ������ˮ��Ȼ����˲ſ��Խ������Ե����������ܵ��������ʷ��롣
(2)���ܽ�ʱ�ò�������������ܽ⣬����ʱ�ò���������������ʱ�ò���������ʹҺ�����Ⱦ��ȡ�
(3)����������������ɫ��ζ���壬˵����Һ�к���![]() ��
��![]() �ļ�����Ҫ�õ�������Ba2+Ϊ�˱���
�ļ�����Ҫ�õ�������Ba2+Ϊ�˱���![]() �ĸ��ţ�����Ӧ��������ϡ������ټ���BaCl2��Һ�����а�ɫ�������ɣ�˵����Һ�д���
�ĸ��ţ�����Ӧ��������ϡ������ټ���BaCl2��Һ�����а�ɫ�������ɣ�˵����Һ�д���![]() ��Cl����ʱӦ��AgNO3��Һ����ԭ��Һ�д���
��Cl����ʱӦ��AgNO3��Һ����ԭ��Һ�д���![]() ��
��![]() �����ȼ���������Ba(NO3)2��Һ���ټ���(�����ữ��)AgNO3��Һ�����а�ɫ�������ɣ�˵��ԭ��Һ�д���Cl��
�����ȼ���������Ba(NO3)2��Һ���ټ���(�����ữ��)AgNO3��Һ�����а�ɫ�������ɣ�˵��ԭ��Һ�д���Cl��

 ������ÿ�ʱ��ҵϵ�д�
������ÿ�ʱ��ҵϵ�д�����Ŀ����úΪԭ�Ͽɺϳ�һϵ��ȼ�ϡ�
��1����֪����2H2��g��+O2��g��= 2H2O��g����H=��483.6kJ/mol
��CH3OH(g)+H2O(g)=CO2(g)+3H2(g)��H=��49.0kJ/mol
��д���״�ȼ������H2O��g�����Ȼ�ѧ����ʽ_________;
��2����1L�ܱ������м���2mol CO��4mol H2�����ʵ��Ĵ��������£�������Ӧ��2CO��g��+4H2��g��![]() CH3OCH3��l��+H2O��l����H=+71kJ/mol
CH3OCH3��l��+H2O��l����H=+71kJ/mol
�ٸ÷�Ӧ�ܷ�_________�Է����У���ܡ��������ܡ������жϡ���
������������˵���˷�Ӧ�ﵽƽ��״̬����_________��
a����������ƽ����Է����������ֲ���
b��CO��H2��ת�������
c��CO��H2������������ֲ���
d�����������ܶȱ��ֲ���
e��1mol CO���ɵ�ͬʱ��1mol O��H������
��3��CO2��g��+3H2��g��![]() CH3OH��g��+H2O��g����H��0��һ�������£�ij��Ӧ�����в����������±���
CH3OH��g��+H2O��g����H��0��һ�������£�ij��Ӧ�����в����������±���
��Ӧ���� | ��Ӧʱ�� | CO2(mol) | H2(mol) | CH3OH(mol) | H2O(mol) |
���� ���� (T1�桢 2L) | 0min | 2 | 6 | 0 | 0 |
10min | 4.5 | ||||
20min | 1 | ||||
30min | 1 |
��0��10min�ڣ���H2O��g����ʾ�Ļ�ѧ��Ӧ����v(H20)=_________mol/(L��min)
�ڴﵽƽ��ʱ���÷�Ӧ��ƽ�ⳣ��K=_________���÷�����ʾ����ƽ��ʱH2��ת������_________��
���������������������£���30minʱ�ı��¶�ΪT
��4���ü��ѣ�CH3OCH3����Ϊȼ�ϵ�ص�ԭ��,��д���ڼ��Խ����е�ظ�����Ӧʽ_________��