��Ŀ����
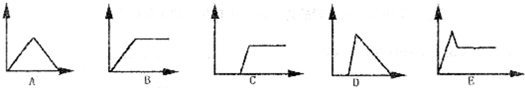
��10�֣�ͼΪһ����AlCl3��Һ�м���NaOH��Һ����Al(OH)3��ɫ������������NaOH�����ʵ���֮��Ĺ�ϵ���ߡ��Իش�

��A��ʱ�Ѳμӷ�Ӧ��AlCl3��NaOH�����ʵ���֮��Ϊ1��_______��
��B����Һ�д��ڵ�Ũ������������_____________�������ӷ��ţ���
����立���һ�ָ��Σ�����Ҫ��ѧ�ɷ�Ϊʮ��ˮ�������NH4Al(SO4)2.12H2O������ε�Ũ��Һ����μ���ŨNaOH��Һ��������һϵ�б仯����֪NH4����AlO2����ˮ��Һ�в��ܴ������棬�ᷢ�����·�Ӧ��NH4����AlO2����H2O��Al(OH)3����NH3����
�Իش�
��1��д�����������ˮ��Һ�е���ķ���ʽ�� ��
��2������μ���NaOH��Һ�Ĺ����У������������У�
����Һ�г��ְ�ɫ���������д̼�����ζ������ų����۰�ɫ�����������ࣻ�ܰ�ɫ������ȫ��ʧ���ݰ�ɫ�������١����ų����ϸ����������ȵ�����ֵ���ȷ˳������Żش� ��
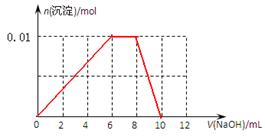
��3������0.01mol NH4Al(SO4)2��Һ����μ���5mol��L��1NaOH��Һ��������ͼ�л������ɳ��������ʵ��������NaOH��Һ����Ĺ�ϵʾ��ͼ��
��10�֣��� 3��2�֣����� Na+��2�֣���
��1��NH4Al(SO4)2��NH4����Al3����2SO42�� ��2�֣���2���٢ۢڢݢ� ��2�֣�
(3) 
��2�֣�
��������
������������ݷ�ӦʽAl3+��3OH��===Al(OH)3����֪��A��ʱ�Ѳμӷ�Ӧ��AlCl3��NaOH�����ʵ���֮��Ϊ1:3��
��B����Һ��ǡ������ƫ�����ƣ�����AlO2��ˮ�⣬Ũ�Ƚ��ͣ����Դ��ڵ�Ũ�����������������ӡ�
��1�����������ˮ��Һ�е���ķ���ʽ��NH4Al(SO4)2��NH4����Al3����2SO42����
��2��������Һ�е������ӿ�֪������μ���NaOH��Һ�Ĺ����У�OH�����Ⱥ�Al3����Ӧ��������������ɫ����������NH4����AlO2����ˮ��Һ�в��ܴ������棬�ᷢ�����·�Ӧ��NH4����AlO2����H2O��Al(OH)3����NH3�������Ե������������ٷ����仯ʱ��OH���ٺ�NH4����Ӧ���ɰ�ˮ������������������ܽ�������������ƫ�����ơ�������ȷ��ʵ�����������Ǣ٢ۢڢݢܡ�
��3�����ݣ�2���з�����֪����ȷ��ͼ����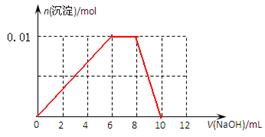
���㣺�����������������Ե��й��жϡ�Ӧ�úͼ���
�����������ۺ���ǿ���ѶȽϴ����������߿��������ǿ��������ע�ضԻ���֪ʶ������̵�ͬʱ�������ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ������˼ά�����ͳ���˼ά���������ѧ���Ĺ淶����������Ӧ��������