��Ŀ����
��9�֣���ȩ(CH3CHO)�ڴ������ڵ������£����Ա��������������ᡣ���ݴ�ԭ�����ʵ���Ƶò����Թ�C���ռ�������������Һ����ͼ��ʾ���Թ�A��װ��40%����ȩˮ��Һ������ͭ��ĩ���Թ�C��װ����������ˮ���ձ�B��װ��ijҺ�壩����֪��60��~80��ʱ��˫�����������������ɷ�����ȩ��������Ӧ����������ʮ���Σ���Ӧ������ȫ���й����ʵķе���£�
��ش��������⣺
��1���Թ�A����60��~80��ʱ��������Ҫ��Ӧ�Ļ�ѧ����ʽΪ��ע����Ӧ������
������_________________________________________��
��2����ͼ��ʾ����ʵ��IJ�ͬ�Σ���Ҫ�����¶ȼ����Թ�A�ڵ�λ�ã���ʵ�鿪ʼʱ��
�ȼ�ˮ�����λ��Ӧ��________�����Թ�A�ڵ���Ҫ��Ӧ��ɺ��¶ȼ�ˮ�����λ��Ӧ��_______��
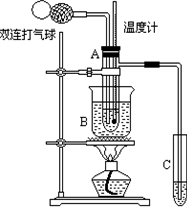
��3���ձ�B��ʢװ��Һ�������_______��
��4���Թ�C���ռ������Ǵֲ�Ʒ�������һ���ᴿ���ɲ��õķ����ǣߣߣߣߣߣ�
�������ᴿ��IJ�Ʒ�����������н��вⶨ�����ʺɱ�����ǣߣߣߣߣ�����ֵ����
���������ں˴Ź������н��вⶨ����˴Ź��������Уߣ��ַ壬�����֮��Ϊ�ߣߡ�
| ���� | ��ȩ | ���� | ���� | �Ҵ� | ˮ |
| �е�/�� | 20.8 | 117.9 | 290 | 78.2 | 100 |
��1���Թ�A����60��~80��ʱ��������Ҫ��Ӧ�Ļ�ѧ����ʽΪ��ע����Ӧ������
������_________________________________________��
��2����ͼ��ʾ����ʵ��IJ�ͬ�Σ���Ҫ�����¶ȼ����Թ�A�ڵ�λ�ã���ʵ�鿪ʼʱ��
�ȼ�ˮ�����λ��Ӧ��________�����Թ�A�ڵ���Ҫ��Ӧ��ɺ��¶ȼ�ˮ�����λ��Ӧ��_______��

��3���ձ�B��ʢװ��Һ�������_______��
��4���Թ�C���ռ������Ǵֲ�Ʒ�������һ���ᴿ���ɲ��õķ����ǣߣߣߣߣߣ�
�������ᴿ��IJ�Ʒ�����������н��вⶨ�����ʺɱ�����ǣߣߣߣߣ�����ֵ����
���������ں˴Ź������н��вⶨ����˴Ź��������Уߣ��ַ壬�����֮��Ϊ�ߣߡ�
��1��2CH3CHO+O2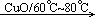 2CH3COOH��
2CH3COOH��
��2�������Թ�A�ķ�ӦҺ�С��Թ�A��֧�ܿڴ���
��3�����͡�����4������60��2��3��1��1��3��
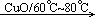 2CH3COOH��
2CH3COOH����2�������Թ�A�ķ�ӦҺ�С��Թ�A��֧�ܿڴ���
��3�����͡�����4������60��2��3��1��1��3��
��1���ڼ��Ȳ��дӣ���������������ȩ�ɷ���������Ӧ�������ᣬ����ʽΪ2CH3CHO+O2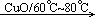 2CH3COOH��
2CH3COOH��
��2����Ӧ��ʼ�Σ���Ҫ������Һ���¶ȣ�����Ӧ������Һ�С�����Ӧ��������Ҫͨ������������ᣬ������Ҫ�����������¶ȣ�����Ӧ�����Թ�A��֧�ܿڴ���
��3����Ϊ����ķе����100�棬�����ձ���Һ����¶Ⱦͱ����������ķе㣬���ݱ������ݿ�֪��Ҫ�ø��͡�
��4�����ɵ���������û�з�Ӧ����ȩ������ȩ�ķе������IJ��ܴ����Կ��Լ���ͨ������õ����ᡣ��������ķ���ʽCH3COOH��֪�ʺɱ��������60.��������к���2�в�ͬ���͵���ԭ�ӣ��������Ϊ3��1��1��3��
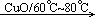 2CH3COOH��
2CH3COOH����2����Ӧ��ʼ�Σ���Ҫ������Һ���¶ȣ�����Ӧ������Һ�С�����Ӧ��������Ҫͨ������������ᣬ������Ҫ�����������¶ȣ�����Ӧ�����Թ�A��֧�ܿڴ���
��3����Ϊ����ķе����100�棬�����ձ���Һ����¶Ⱦͱ����������ķе㣬���ݱ������ݿ�֪��Ҫ�ø��͡�
��4�����ɵ���������û�з�Ӧ����ȩ������ȩ�ķе������IJ��ܴ����Կ��Լ���ͨ������õ����ᡣ��������ķ���ʽCH3COOH��֪�ʺɱ��������60.��������к���2�в�ͬ���͵���ԭ�ӣ��������Ϊ3��1��1��3��

��ϰ��ϵ�д�
�����Ŀ