��Ŀ����
����Ŀ��ijͬѧ�������ʵ�鷽�����Է���KCl��BaCl2���ֹ�������Իش��������⣺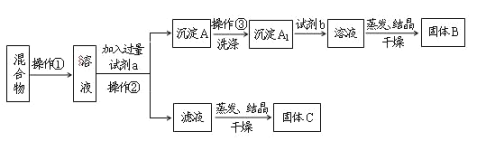
��ѡ�Լ���Na2SO4��Һ��K2CO3��Һ��K2SO4��Һ������
��1���Լ�a��____���Լ�b��___��
��2�������Լ�a��������Ӧ�Ļ�ѧ����ʽΪ____�������Լ�b��������Ӧ�Ļ�ѧ����ʽΪ_____��
��3���÷����ܷ�ﵽʵ��Ŀ�ģ�____���������������������������ܣ�Ӧ��θĽ��������ܣ����ʲ��ûش�_____��
��4����Ҫ�ⶨԭ�������BaCl2����������������Ҫȷ���������������⣬���ٻ�Ҫ��õ�������____��������
���𰸡�K2CO3 HCl K2CO3��BaCl2=BaCO3����2KCl BaCO3��2HCl=BaCl2��CO2����H2O ��(����) Ӧ�ڲ����ڵ���Һ�м������������������ᾧ ����A1�����B
��������
����KCl��BaCl2���ֹ��������������ˮ��Ȼ��������K2CO3ʹBaCl2ת��Ϊ���������˺�����������������BaCl2��Һ�����������ᾧ�������ɵù���BaCl2��������������ҺΪKCl��K2CO3�Ļ��������ᾧ�õ�����CΪKCl��K2CO3��Ӧ�����������ɵ�KCl������AΪBaCO3��ϴ�Ӻ����ᣬ�����õ�����BΪBaCl2��
��1��������Ϊ�����Һ��ķ��룬Ϊ���˲����������ܽ��������K2CO3ʹBaCl2ת��Ϊ���������Լ�aΪK2CO3��BaCO3+2H��=Ba2��+CO2��+H2O�������Լ�b��BaCO3����ת��ΪBaCl2��Һ���Լ�bΪ���
��2�������Լ�a��������Ӧ�Ļ�ѧ����ʽΪ K2CO3��BaCl2=BaCO3����2KCl�������Լ�b��������Ӧ�Ļ�ѧ����ʽΪBaCO3��2HCl=BaCl2��CO2����H2O��
��3���÷������ܴﵽʵ��Ŀ�ģ�������������ҺΪKCl��K2CO3�Ļ��������ᾧ�õ�����CΪKCl��K2CO3��Ӧ�����������ɵ�KCl����Ӧ�ڲ����ڵ���Һ�м������������������ᾧ��
��4���ⶨ�Ȼ����������������ɼ���̼�ᱵ�������Ȼ��������������õ�����A1�����B��������

 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д�����Ŀ��ijѧ����0.200 0 mol��L��1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ��������£�
��������ˮϴ�Ӽ�ʽ�ζ��ܣ�������ע��NaOH��Һ����0���̶������ϣ�
�ڹ̶��õζ��ܲ�ʹ�ζ��ܼ��촦����Һ�壻
�۵���Һ������0������0���̶������£������¶�����
����ȡ20.00 mL����Һע��ྻ�Ļ�������������ˮ����ƿ�У�������3�η�̪��Һ��
���ñ�Һ�ζ����յ㣬���µζ���Һ�������
��ش��������⣺
��1�����ϲ����д������______(����)�����ⶨ���ƫ�ߣ���ԭ�������________(����ĸ)��
A.���Ʊ���Һ�Ĺ���NaOH�л���KOH����
B.�ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�����������ȷ
C.ʢװδ֪Һ����ƿ������ˮϴ��������δ֪Һ��ϴ
D.����ı�NaOH��Һ���ʵ���Ũ��ƫ��
��2���жϵζ��յ��������_____________________________________��
��3����ͼ��ij�εζ�ʱ�ĵζ����е�Һ�棬�����Ϊ________mL��

��4�������������ݣ��������������Ũ�ȣ�________mol��L��1��
�ζ����� | �������(mL) | ���ռ���Һ���(mL) | |
�ζ�ǰ���� | �ζ������ | ||
��һ�� | 20.00 | 0.40 | 20.40 |
�ڶ��� | 20.00 | 2.00 | 24.10 |
������ | 20.00 | 4.00 | 24.00 |