��Ŀ����
��ѧһ���ʽṹ���������������ǻ���ɫ��״�ᾧ����ṹ��ͼ��ʾ����������ˮ�����ڵ����ᡢ����̼�������л��ܼ����ڷ��ڵ�NaOHϡ��Һ�л�Ѹ��ˮ�⡣�ش���������:

��1��Se��S����һ����ͬ����Ԫ�أ����������Ų�ʽΪ_________��
��2����һ������:S_________(�>����<����=������ͬ)P���縺�ԣ�S_________P��
��3��������������Pԭ�Ӳ�ȡ_________�ӻ�����PO3-��Ϊ�ȵ�����Ļ�������ӵĻ�ѧʽΪ_________��
��4������̼����_________ (����ԡ��Ǽ��ԡ�)���ӡ�
��5����NA��ʾ�����ӵ���������ֵ��0��1mol�����������к��еŵ��Ӷ���Ϊ_________��
��6����������(HN3)�ڳ�������һ��Һ�壬�е�ϸߣ�Ϊ308��8 K����Ҫԭ����_________��
��7���������ƾ���NaCl�ͽṹ���侧����Na+��OH-֮��ľ���Ϊacm��������Na+����λ��Ϊ_________����NA��ʾ�����ӵ���������ֵ��NaOH���ܶ�Ϊ_________g��cm-3��
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ


 B. ���������ӵĵ���ʽ��
B. ���������ӵĵ���ʽ��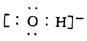
 D. ������Ϊ146��������Ϊ92����(U)ԭ��
D. ������Ϊ146��������Ϊ92����(U)ԭ��


 CH3OH(l) ��H=?
CH3OH(l) ��H=?
