��Ŀ����
��10�֣�Fe��Al�����ֳ��õĽ������ֽ����ǰ�һ������������ɾ��ȵĻ���
��1��ȡһ�������ĸû��������м���������NaOH��Һ���������������ڱ�״����Ϊn L����Ӧ�����ӷ���ʽΪ ��
�������Al�����ʵ���Ϊ ���ú���ĸ����ѧʽ��ʾ����
��2����ȡ��ͬ�����ĸû��������м���������ϡ���ᣬ����ȫ���ܽ⣬�������������ڱ�״����ΪmL����Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ ���������Fe������Ϊ ���ú���ĸ����ѧʽ��ʾ����
��1��2Al+2OH-+2H2O===2AlO2-+3H2����n/33.6 mol����2�֣�
��2��m/11.2 mol �� 2.5��m��n��g����3�֣�
��������(1)��������ֻ�����źͼ���Һ��Ӧ����������������������������������
��2�������ķ�ӦΪ��2Al+2OH-+2H2O===2AlO2-+3H2�� Fe��2H��=Fe2����H2��
�ɷ�Ӧ����ʽ�ɿ���ÿ����1mol��������2mol���ӵ�ת�ƣ��ʷ�Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ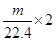 = m/11.2 mol��
= m/11.2 mol��
�ɵ��ӵĵ�ʧ�غ��֪������������Ϊx g��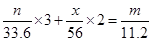 �����x=2.5��m��n��g
�����x=2.5��m��n��g

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�