��Ŀ����
��8�֣�������CO��HCOOH��HOOC��CHO����ȩ�ᣩ�ֱ�ȼ��ʱ�����ĵ����������ɵĶ�����̼������ȶ���1��2�������ߵķ���ʽ���Էֱ��ǣ�CO����H2O���ͣ�CO��2��H2O����Ҳ����˵��ֻҪ����ʽ���ϣ�CO��n(H2O)m�� n��m��Ϊ���������ĸ����л������ȼ��ʱ���ĵ����������ɵĶ�����̼�����������1��2��
����һЩֻ��C��H��O����Ԫ�ص��л������ȼ��ʱ���ĵ����������ɵĶ�����̼���������3��4��
��1����Щ�л����У���Է���������С�Ļ�������________��
��2��ij����̼ԭ������ͬ�������л�������ǵ���Է��������ֱ�Ϊa��b��a��b������a-b�ض���________������һ�����֣�����������
��3������Щ�л�������һ�ֻ�����������������ǻ���ȡ0.2625 g���л���ǡ���ܸ�25.00 mL 0.100 mol��L-1 NaOH��Һ��ȫ�кͣ��ɴ˿��Լ����֪�û��������Է���������__________________�������Ƶ����ķ���ʽӦΪ____________________��
����һЩֻ��C��H��O����Ԫ�ص��л������ȼ��ʱ���ĵ����������ɵĶ�����̼���������3��4��
��1����Щ�л����У���Է���������С�Ļ�������________��
��2��ij����̼ԭ������ͬ�������л�������ǵ���Է��������ֱ�Ϊa��b��a��b������a-b�ض���________������һ�����֣�����������
��3������Щ�л�������һ�ֻ�����������������ǻ���ȡ0.2625 g���л���ǡ���ܸ�25.00 mL 0.100 mol��L-1 NaOH��Һ��ȫ�кͣ��ɴ˿��Լ����֪�û��������Է���������__________________�������Ƶ����ķ���ʽӦΪ____________________��
��1��C2H2O2 ��2��18 ��3��210 ��C2O��3��H2O��5
ȼ��ʱ���ĵ����������ɵĶ�����̼���������1��2����������������л������ʽ���ǣ�CO��n(H2O)m��n��m��Ϊ����������ͨʽʵ�ʿɷ�Ϊ�����ּ���CO��n�ͣ�H2O��m����H2O��m��mH2O�����в���������������CO��n��nCO2�Ĺ������������������ʵ���ǡ�������ɶ�����̼��һ�롣�ɼ����ĵ����������ɵĶ�����̼�������Ϊ1��2�����ڣ�CO��n��nCO2��ɵġ�Ҫ�����������������ɵĶ�����̼�����ʵ���֮��Ϊ3��4��������������
4COx+3O2 4CO2��������ԭ���غ��x=
4CO2��������ԭ���غ��x=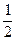 ����C2O��ʽ����
����C2O��ʽ����
���Ը��л���ͨʽΪ��C2O��n��H2O��m(n��m��Ϊ������)��
��1������Է���������С���л������ʽΪC2H2O2��n=1��m=1����
��2������Ŀָ��̼ԭ�Ӹ�����ͬ����n���䣬��m��1(m��Ϊ������)������a-b�ض���18�ı�����
��3�����������軯������2�����ǻ����������Ħ�������ǣ�
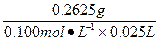 ��2="210" g��mol-1
��2="210" g��mol-1
����n=2ʱ����C2O��2��Է���������80����210��ȥ80��130������18����������n=3ʱ��m=5������ʽӦΪ��C2O��3��H2O��5��
4COx+3O2
 4CO2��������ԭ���غ��x=
4CO2��������ԭ���غ��x=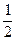 ����C2O��ʽ����
����C2O��ʽ�������Ը��л���ͨʽΪ��C2O��n��H2O��m(n��m��Ϊ������)��
��1������Է���������С���л������ʽΪC2H2O2��n=1��m=1����
��2������Ŀָ��̼ԭ�Ӹ�����ͬ����n���䣬��m��1(m��Ϊ������)������a-b�ض���18�ı�����
��3�����������軯������2�����ǻ����������Ħ�������ǣ�
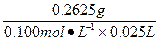 ��2="210" g��mol-1
��2="210" g��mol-1����n=2ʱ����C2O��2��Է���������80����210��ȥ80��130������18����������n=3ʱ��m=5������ʽӦΪ��C2O��3��H2O��5��

��ϰ��ϵ�д�
 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�
�����Ŀ
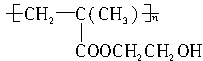 ,�����������������۾��IJ��ϡ���д�������йط�Ӧ�Ļ�ѧ����ʽ��
,�����������������۾��IJ��ϡ���д�������йط�Ӧ�Ļ�ѧ����ʽ��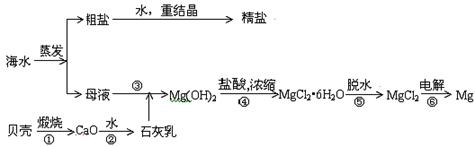
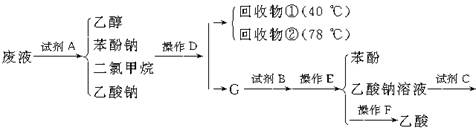
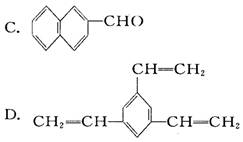
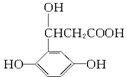 �������ܷ����ķ�Ӧ�У���ȡ����Ӧ���ڼӳɷ�Ӧ������ȥ��Ӧ����ˮ�ⷴӦ����������Ӧ���Ӿ۷�Ӧ��������ȷ���ǣ� ��
�������ܷ����ķ�Ӧ�У���ȡ����Ӧ���ڼӳɷ�Ӧ������ȥ��Ӧ����ˮ�ⷴӦ����������Ӧ���Ӿ۷�Ӧ��������ȷ���ǣ� ��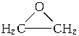 �����Ҷ������ã�����һ����Ӧ������������������ȿ�����һϵ��˫�����Ż������Ϊ�����Ҷ����ѣ��䷴Ӧԭ���ɱ�ʾΪ��
�����Ҷ������ã�����һ����Ӧ������������������ȿ�����һϵ��˫�����Ż������Ϊ�����Ҷ����ѣ��䷴Ӧԭ���ɱ�ʾΪ��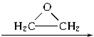 HO��CH2��CH2��O��CH2��CH2��OH
HO��CH2��CH2��O��CH2��CH2��OH