��Ŀ����
��9�֣�0.2mol�л���A��0.4mol O2���ܱ�������ȼ�պ�IJ���ΪCO2��CO��H2O��g����ȼ�պ����Щ���ᆳ��Ũ�������������10.8g����ͨ�����ȵ�CuO��ַ�Ӧ������������3.2g�����������ͨ����ʯ�ұ���ȫ���գ���������17.6g��
��1���ƶϸ��л���A�ķ���ʽ��
��2����0.2mol���л���A����������Ʒ�Ӧ��ų�4.48L H2����״���£�����ȷ�����л���Ľṹ��ʽ��
��1��������ˮ�����ʵ�����n(H2O)��10.8g��18g/mol��0.6mol
CO������ͭ��Ӧ�ķ���ʽΪ
CO��CuO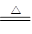 CO2��Cu
��m��
CO2��Cu
��m��
1mol 1mol 16g
0.2mol 0.2mol 3.2g
���Է�Ӧ�����ɵ�CO2��17.6g��44g/mol��0.2mol��0.2mol
���л���A�к��е���ԭ�ӵ����ʵ�����0.6mol��0.2mol��0.2mol��2��0.4mol��2��0.4mol
����A��C��H��O��ԭ�Ӹ���֮����0.4�U1.2�U0.4��1�U3�U1
�����ʽΪCH3O
����C��H����֮��Ϊ1�U3��ֻ��������
����A�ķ���ʽΪC2H6O2
��2��4.48L������0.2mol��˵����Ӧ�к���2���ǻ�
��˽ṹ��ʽΪHOCH2CH2OH
�������������л������ʽ���ṹ��ʽ���жϡ�

 ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�

 ���������ŵ�������
���������ŵ�������
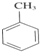 +3HNO3
+3HNO3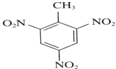 +3H2O��
+3H2O��
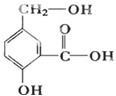 ij�л���A�ķ��ӽṹ��ʽ��ͼ��
ij�л���A�ķ��ӽṹ��ʽ��ͼ��