��Ŀ����
������ɸ������Ϊ������������������������Ը���������Ҫ�ɷ�ΪAl2O3��SiO2�ȣ���ʯ��ʯ����ˮΪԭ������������ɸ���Ĺ���������ͼ��ʾ���ش��������⣺

��1����ˮ�к�Mg2+��Ca2+��SO2-4���ʣ�Ϊ��ȡNaOH���������ӱ����ȥ�������ӡ�ʱ�谴һ��˳����������Լ����ٹ�����NaOH��Һ���ڹ�����Na2CO3��Һ��������������ܹ�����BaCl2��Һ����ȷ������˳����______��ҪʹCa2+��ȫ����������Һ��c��Ca2+����1��10-5mol/L������Һ��c��CO2-3��Ӧ��С��______��֪ Ksp��CaCO3��=2.9��10-9����
��2������M������������ҺN����B�����ӷ���ʽ��______��
��3��д��������A����ˮ������Һ�и�����Ũ�ȴӴ�С��˳��Ϊ______��
��4�����������п���ѭ�����õ�������______��
��5����֪��ij��������Al2O3����������Ϊ35%����1t�������������Ͽ��Ƶû�ѧʽΪNa2O?Al2O3?2SiO2?9/2H2O��Al2O3����������Ϊ28%���ķ���ɸ______t��
�⣺��1�����ʳ��ӵ�ԭ���ǡ��������������֡���ԭ���������˽���������Ŀ�ģ����ܷ������˳���������NaOH��Һ��Ϊ�˳�ȥMg2+�����������BaCl2��Һ��Ϊ�˳�ȥSO42-�����������Na2CO3��Һ��Ϊ�˳�ȥ������Ca2+�Ͷ����Ba2+��Ȼ����й��ˣ���ȥ���ɵ������Ȼ��������������ᣬ��ȥ�����CO32-�͵�����Һ������ԣ������������ᾧ�õ�������NaCl���壮���������˳���Ҫ����Na2CO3��Һ������BaCl2��Һ֮����룬���������ᣬ����̼��Ƶ��ܶȻ��������㣬ҪʹCa2+��ȫ������Ӧ��
c��Ca2+����c��CO2-3����2.9��10-9����c��CO2-3����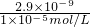 =2.9��10-4mol/L���ʴ�Ϊ���ܢ٢ڢۻ�٢ܢڢۣ�2.9��10-4mol/L��
=2.9��10-4mol/L���ʴ�Ϊ���ܢ٢ڢۻ�٢ܢڢۣ�2.9��10-4mol/L��
��2��ʯ��ʯ�������ɵ�����MΪCO2�����������պ�IJ�����Ҫ��SiO2��Al2O3����NaOH���˺�õ�����ҺN��Ҫ����NaAlO2��Na2SiO3������ͨ������CO2������Ӧ�ķ���ʽΪ��NaAlO2+CO2+2H2O=Al��OH��3��+NaHCO3��Na2SiO3+2CO2+2H2O=H2SiO3+2NaHCO3�����ӷ���ʽΪ��SiO32-+2CO2+2H2O�TH2SiO3��+2HCO3-��AlO2-+2H2O+CO2�TAl��OH��3��+HCO3-��
�ʴ�Ϊ��SiO32-+2CO2+2H2O�TH2SiO3��+2HCO3-��AlO2-+2H2O+CO2�TAl��OH��3��+HCO3-��
��3��̼����ˮ��ʼ��ԣ�����CO32-���Ӵ�������ˮ�⣬�ҵ�һ��ˮ����ڵڶ���ˮ�⣬���У�c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+����
�ʴ�Ϊ��c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+����
��4��ʯ��ʯ�ֽ����ɶ�����̼���壬���ڹ�ҵ���ɣ����ɵ���ҺN�к���̼�����ƣ����Ⱥ������ɶ�����̼���壬��ѭ�����ã��ʴ�Ϊ��CO2��
��5�������������������غ���㣬�����ɷ���ɸ������Ϊx������1t��35%=28%��xt��x=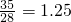 t��
t��
�ʴ�Ϊ��1.25��
��������1�����ʳ��ӵ�ԭ���ǡ��������������֡���ԭ����ע��һ�㣬�Ǿ�����������������Na2CO3��Һ��Ϊ�˳�ȥ������Ca2+�Ͷ����Ba2+��Ȼ����й��ˣ���ȥ���ɵ������Ȼ��������������ᣬ��ȥ�����CO32-�͵�����Һ������ԣ������������ᾧ�õ�������NaCl���壻����̼��Ƶ��ܶȻ��������㣻
��2��ʯ��ʯ�������ɵ�����MΪCO2�����������պ�IJ�����Ҫ��SiO2��Al2O3����NaOH���˺�õ�����ҺN��Ҫ����NaAlO2��Na2SiO3���Դ���д���ӷ���ʽ��
��3��̼����ˮ��ʼ��ԣ�����CO32-���Ӵ�������ˮ�⣬�ҵ�һ��ˮ����ڵڶ���ˮ�⣬�Դ��ж�����Ũ�ȴ�С˳��
��4��ʯ��ʯ�ֽ����ɶ�����̼���壬���ڹ�ҵ���ɣ����ɵ���ҺN�к���̼�����ƣ����Ⱥ������ɶ�����̼���壬��ѭ�����ã�
��5�������������������غ���㣮
������������һ���ԡ�����ɸ�����Ʊ�Ϊ�����Ļ�ѧ���������⣬����ȡ�Ľ��£�����������ǻ�ѧ����֪ʶ�������������ʵ�ʣ��Խ����ѧʵ��������˼·�������ʣ�ʹ�����龳��ʵ��������ֻ�ѧ��STSE�Ĺ�ϵ������ѧ�Ա�ɫ��������ѧ��������ϵʵ�ʣ�ѧ�����õ�ѧϰ�ۣ����⿼���֪ʶ��࣬Ҳ�dz���˼ά�����ϴ���Ŀ�Ի�ѧ֪ʶ�������Ӧ��֪ʶ������������������й������Ǩ����������Ҫ��ϸߣ�������ӱ���Ķ������ܿ���ѧ�����Ķ����������ϵ��ռ�����������ͬʱ��ѧ���������������������⡢������⡢���ֱ���ȷ����������Ҫ��dz��ߣ���һ���ۺ������⣬��ѧ���Ժ��ѧϰ���������õ�ָ�����ã�
c��Ca2+����c��CO2-3����2.9��10-9����c��CO2-3����
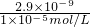 =2.9��10-4mol/L���ʴ�Ϊ���ܢ٢ڢۻ�٢ܢڢۣ�2.9��10-4mol/L��
=2.9��10-4mol/L���ʴ�Ϊ���ܢ٢ڢۻ�٢ܢڢۣ�2.9��10-4mol/L����2��ʯ��ʯ�������ɵ�����MΪCO2�����������պ�IJ�����Ҫ��SiO2��Al2O3����NaOH���˺�õ�����ҺN��Ҫ����NaAlO2��Na2SiO3������ͨ������CO2������Ӧ�ķ���ʽΪ��NaAlO2+CO2+2H2O=Al��OH��3��+NaHCO3��Na2SiO3+2CO2+2H2O=H2SiO3+2NaHCO3�����ӷ���ʽΪ��SiO32-+2CO2+2H2O�TH2SiO3��+2HCO3-��AlO2-+2H2O+CO2�TAl��OH��3��+HCO3-��
�ʴ�Ϊ��SiO32-+2CO2+2H2O�TH2SiO3��+2HCO3-��AlO2-+2H2O+CO2�TAl��OH��3��+HCO3-��
��3��̼����ˮ��ʼ��ԣ�����CO32-���Ӵ�������ˮ�⣬�ҵ�һ��ˮ����ڵڶ���ˮ�⣬���У�c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+����
�ʴ�Ϊ��c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+����
��4��ʯ��ʯ�ֽ����ɶ�����̼���壬���ڹ�ҵ���ɣ����ɵ���ҺN�к���̼�����ƣ����Ⱥ������ɶ�����̼���壬��ѭ�����ã��ʴ�Ϊ��CO2��
��5�������������������غ���㣬�����ɷ���ɸ������Ϊx������1t��35%=28%��xt��x=
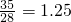 t��
t���ʴ�Ϊ��1.25��
��������1�����ʳ��ӵ�ԭ���ǡ��������������֡���ԭ����ע��һ�㣬�Ǿ�����������������Na2CO3��Һ��Ϊ�˳�ȥ������Ca2+�Ͷ����Ba2+��Ȼ����й��ˣ���ȥ���ɵ������Ȼ��������������ᣬ��ȥ�����CO32-�͵�����Һ������ԣ������������ᾧ�õ�������NaCl���壻����̼��Ƶ��ܶȻ��������㣻
��2��ʯ��ʯ�������ɵ�����MΪCO2�����������պ�IJ�����Ҫ��SiO2��Al2O3����NaOH���˺�õ�����ҺN��Ҫ����NaAlO2��Na2SiO3���Դ���д���ӷ���ʽ��
��3��̼����ˮ��ʼ��ԣ�����CO32-���Ӵ�������ˮ�⣬�ҵ�һ��ˮ����ڵڶ���ˮ�⣬�Դ��ж�����Ũ�ȴ�С˳��
��4��ʯ��ʯ�ֽ����ɶ�����̼���壬���ڹ�ҵ���ɣ����ɵ���ҺN�к���̼�����ƣ����Ⱥ������ɶ�����̼���壬��ѭ�����ã�
��5�������������������غ���㣮
������������һ���ԡ�����ɸ�����Ʊ�Ϊ�����Ļ�ѧ���������⣬����ȡ�Ľ��£�����������ǻ�ѧ����֪ʶ�������������ʵ�ʣ��Խ����ѧʵ��������˼·�������ʣ�ʹ�����龳��ʵ��������ֻ�ѧ��STSE�Ĺ�ϵ������ѧ�Ա�ɫ��������ѧ��������ϵʵ�ʣ�ѧ�����õ�ѧϰ�ۣ����⿼���֪ʶ��࣬Ҳ�dz���˼ά�����ϴ���Ŀ�Ի�ѧ֪ʶ�������Ӧ��֪ʶ������������������й������Ǩ����������Ҫ��ϸߣ�������ӱ���Ķ������ܿ���ѧ�����Ķ����������ϵ��ռ�����������ͬʱ��ѧ���������������������⡢������⡢���ֱ���ȷ����������Ҫ��dz��ߣ���һ���ۺ������⣬��ѧ���Ժ��ѧϰ���������õ�ָ�����ã�

��ϰ��ϵ�д�
 ��ѧ����ϵ�д�
��ѧ����ϵ�д�
�����Ŀ
