��Ŀ����
����Ŀ�����ʵ������ṹ���������ʵ�������仯���ش��������⣺
��1����̬��ԭ�Ӽĵ����Ų�ʽΪ[Ar]___��
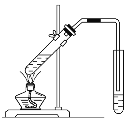
��2�����������ڰ�ˮ�γ�[Ni(NH3)6]SO4��ɫ��Һ��
��[Ni(NH3)6]SO4�������ӵ����幹����___��
����[Ni(NH3)6]2+��Ni2+��NH3֮���γɵĻ�ѧ����Ϊ___���ṩ�µ��ӶԵijɼ�ԭ����___��
�۰��ķе�����(PH3)��ԭ����___��PH3������Pԭ�ӵ��ӻ��������Ϊ___��
��3��ͭ��(SCN)2��Ӧ����Cu(SCN)2��1mol(SCN)2�к�����������ĿΪ___��
��4����Ȼ��������ɸ��ӣ������ӵĻ����ṹ��Ԫ��[SiO4]�����壬��ͼ(a)��ͨ�����ö��������ӿ��γ���״����״�Ƚṹ��ͼ(b)Ϊһ������˫���ṹ�Ķ��������ö������Ļ�ѧʽΪ___(��n�����ۺ϶�)��

��5���������γɵ�ij�ֻ����ᄃ���ṹ��������ͼ��ʾ���û�����Ļ�ѧʽ��___���侧���ܶȵļ������ʽΪ___g��cm-3(�����ӵ�������ֵ��NA��ʾ)��
���𰸡�3d64s2 �������� ��λ�� N NH3���Ӽ���γ���� sp3 4NA ![]() MnI2
MnI2 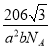
��������
(1)��̬��ԭ�Ӻ�����26�����ӣ����������Ų�ʽΪ1s22s22p63s23p63d64s2���ĵ����Ų�ʽΪ[Ar]3d64s2���ʴ�Ϊ��3d64s2��
(2)��SO42-����ԭ�ӵļ۵��Ӷ���Ϊ![]() ��S�µ��Ӷԣ����幹��Ϊ�������壬�ʴ�Ϊ���������壻
��S�µ��Ӷԣ����幹��Ϊ�������壬�ʴ�Ϊ���������壻
��[Ni(NH3)6]2+Ϊ�����ӣ�Ni2+��NH3֮��Ϊ��λ��������NH3���ṩ�µ��ӶԵ�ΪN���ʴ�Ϊ����λ����N��
��NH3���Ӽ����������ʷе��PH3�ߣ�NH3��N��һ���µ��Ӷԣ����幹��Ϊ�����Σ����NH3Ϊ���Է��ӣ�N���ӻ������Ϊ3+1=4���ӻ�����Ϊsp3���ʴ�Ϊ��NH3���Ӽ���γ������sp3��
(3)(SCN)2�Ľṹ��ʽΪN��C��S��S��C��N����1mol(SCN)2�к�����������ĿΪ4NA���ʴ�Ϊ��4NA��
(4)ͼ(b)Ϊһ��������״�ṹ�Ķ�������ͼ(a)��һ��SiO44-������ṹ��Ԫ��������3����ԭ�ӵĹ�����Ϊ![]() ���������ṹ��Ԫ�к���2+4��
���������ṹ��Ԫ�к���2+4��![]() =4���裬��ԭ����Ŀ=2+4��
=4���裬��ԭ����Ŀ=2+4��![]() +6��
+6��![]() +4=11����ö�����εĻ�ѧʽΪ
+4=11����ö�����εĻ�ѧʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(5)���ݾ�̯����֪��ÿ�������к���8![]() =1�������ӣ�2�������ӣ��û�����Ļ�ѧʽ��MnI2�������ܶ�
=1�������ӣ�2�������ӣ��û�����Ļ�ѧʽ��MnI2�������ܶ�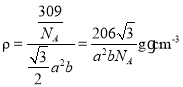 ���ʴ�Ϊ��MnI2��
���ʴ�Ϊ��MnI2��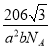 ��
��

����Ŀ��ѡ��װ�ã����ʵ�顣
|
|
|
|
�� | �� | �� | �� |
��1������ˮ��ֲ���ͣ�ѡ��___������ţ���ͬ����
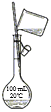
��2������100mL0.1mol��L-1NaOH��Һ��ѡ��___��
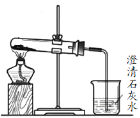
��3������Na2CO3��NaHCO3���壬ѡ��__��
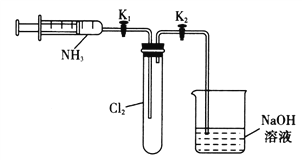
��4�������ᡢ�Ҵ���Ũ�����Ʊ�����������ѡ��__��
����Ŀ��ֱ���ŷź�SO2���������γ����꣬Σ�������������Ƽ�ѭ�������ѳ������е�SO2������Һ����SO2�Ĺ����У�pH��n(Na2SO3):n(NaHSO3)�仯��ϵ���±���
n(Na2SO3):n(NaHSO3) | 91:9 | 1:1 | 9:91 |
pH | 8.2 | 7.2 | 6.2 |
��1�����ϱ��ж�NaHSO3��Һ��_____�ԣ��Խ���ԭ��______��
��2��pH��8.2������Һ����ˮ�������c(OH��)______0.1molL-1NaOH��Һ����ˮ���������c(OH��)������������������������������
��3����pH��8.2������Һ���ɵõ��������Ҫ�ɷ���______��
��4���������������pH��NaOH��Һ��Na2SO3��Һ�ֱ��ˮϡ��m����n����ϡ�ͺ�����ҺpH����ȣ���m______n(������������������������)��
��5��25��ʱ��������ҺΪ����ʱ����Һ������Ũ�ȹ�ϵ��ȷ����______(ѡ����ĸ)��
A c(Na��)��2c(SO![]() )��c(HSO
)��c(HSO![]() )
)
B c(Na��)��c(HSO![]() )��c(SO
)��c(SO![]() )��c(H��)��c(OH��)
)��c(H��)��c(OH��)
C c(Na��)��c(H��)��c(SO![]() )��c(HSO
)��c(HSO![]() )��c(OH��)
)��c(OH��)