��Ŀ����
����Ŀ��������N2H4����һ����ɫ�Ŀ�ȼҺ�塣��ش��������⣺
��1�������ǻ������Ҫȼ�ϡ���֪��
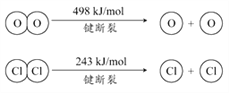
(a) N2H4(l)��ȼ���Ȧ�H1 = �C624.0 kJmol�C1
(b) ![]() ��H2 = �C66.4 kJmol�C1
��H2 = �C66.4 kJmol�C1
(c) ![]() ��H3 = �C28.6 kJmol�C1
��H3 = �C28.6 kJmol�C1
д��N2H4(l)��N2O4(g)��ȼ�����ɵ�����Һ̬ˮ���Ȼ�ѧ����ʽ________________��
��2��N2H4��ʹ��¯�ڱڵ����⣨��Ҫ�ɷ�ΪFe2O3xH2O����ɴ������������Ӷ��ɼ�����¯��ʴ����Ӧ������ÿ����0.1 mol������������ת�Ƶĵ�����Ϊ_________________��
��3������-����ȼ�ϵ�صĵ����ΪKOH��Һ��д���õ�طŵ�ʱ�����ķ�Ӧʽ________��
��4���������Ʊ������ж��֣����ط�������֮һ����KMnO4�Ĵ������£�����CO(NH2)2��NaClO��NaOH��Һ��Ӧ����������ˮ����������,д���÷�Ӧ�Ļ�ѧ����ʽ_________________��
���𰸡� ![]() ��H = �C1124.4 kJmol�C1 6.02��1022 N2H4-4 e��+4OH-=N2��+4H2O CO(NH2)2+NaClO+2NaOH=N2H4+H2O+NaCl+Na2CO3
��H = �C1124.4 kJmol�C1 6.02��1022 N2H4-4 e��+4OH-=N2��+4H2O CO(NH2)2+NaClO+2NaOH=N2H4+H2O+NaCl+Na2CO3
����������1��(a)���Ȼ�ѧ����ʽΪN2H4(l)+ O2(g)=N2(g)+2H2O(l) ��H1 =�C624.0 kJmol�C1�� N2H4(l)��N2O4(g)��ȼ�����ɵ�����Һ̬ˮ���Ȼ�ѧ����ʽΪ(d): 2N2H4(l)+N2O4(g)=3N2(g)+4H2O(l) ��H4������ʽd=2a-(b+2c)��������H4=2��H1-����H2+2��H3��=�C1124.4 kJmol�C1�����Զ�Ӧ���Ȼ�ѧ����ʽΪ��2N2H4(l)+N2O4(g)=3N2(g)+4H2O(l) ��H4=�C1124.4 kJmol�C1��
��2������������ΪFe3O4��Fe2O3xH2O��N2H4��ԭΪFe3O4�����Ļ��ϼ۴�+3�۽��͵�+8/3�ۣ�����1molFe3O4�õ����ӣ�3-8/3����3��1mol=3mol����������0.1 mol����������ת�Ƶĵ�����Ϊ0.1��3��6.02��1023= 6.02��1022��
��3������-����ȼ�ϵ���У������ڸ���ʧȥ��������N2�������ĵ缫��ӦʽΪ��
N2H4-4 e��+4OH-=N2��+4H2O��
��4��KMnO4������������CO(NH2)2��NaClO��NaOH��Һ��Ӧ����������ˮ���������Σ��ݴ˿�д����Ӧ�Ļ�ѧ����ʽΪ��CO(NH2)2+NaClO+2NaOH=N2H4 +H2O+NaCl+Na2CO3��