��Ŀ����
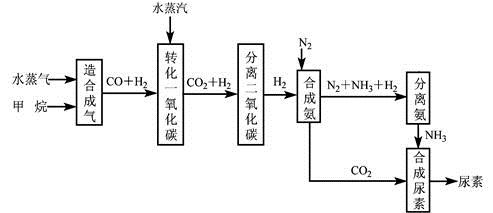
�ҹ��зḻ����Ȼ����Դ������Ȼ��Ϊԭ�Ϻϳ����ص���Ҫ��������ͼ��ʾ(ͼ��ijЩת�����輰������δ�г�)��
��д���пհף�
(1)��֪0.5 mol�����0.5 molˮ������t �棬p k Paʱ����ȫ��Ӧ����һ����̼������(�ϳ���)��������a kJ�������÷�Ӧ���Ȼ�ѧ����ʽ�� ��
(2)���������У���ҵ�Ϸ���H2 ��CO2�����ķ����� ��
A�������ͨ������������Һ��������Һ�м�����
B�������ѹ��ȴ��ʹCO2Һ��
C������ð�ˮϴ��
D�������ͨ�뵽ʯ�ҽ���Ȼ��������չ��壬
(3)Ϊ�˱�֤����˳���ϳɣ��ڿ�������ϳ���֮ǰ����Կ������� ��Ŀ����____________________���ںϳɰ���ʵ�����������У�����ȡ�����ɵİ��ӻ�������з����������������ķ��� ��
(4)������������Դ����������߾���Ч�棬����Ҳ�Ƕ���ᡢ��ȫ���ฺ��ı��֣�����ͼ�е��������Ժ���������Դ��� ��

��д���пհף�
(1)��֪0.5 mol�����0.5 molˮ������t �棬p k Paʱ����ȫ��Ӧ����һ����̼������(�ϳ���)��������a kJ�������÷�Ӧ���Ȼ�ѧ����ʽ�� ��
(2)���������У���ҵ�Ϸ���H2 ��CO2�����ķ����� ��
A�������ͨ������������Һ��������Һ�м�����
B�������ѹ��ȴ��ʹCO2Һ��
C������ð�ˮϴ��
D�������ͨ�뵽ʯ�ҽ���Ȼ��������չ��壬
(3)Ϊ�˱�֤����˳���ϳɣ��ڿ�������ϳ���֮ǰ����Կ������� ��Ŀ����____________________���ںϳɰ���ʵ�����������У�����ȡ�����ɵİ��ӻ�������з����������������ķ��� ��
(4)������������Դ����������߾���Ч�棬����Ҳ�Ƕ���ᡢ��ȫ���ฺ��ı��֣�����ͼ�е��������Ժ���������Դ��� ��
(1)CH4(g)+H2O(g)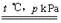 CO(g)+3H2(g)��H="+2a" kJ/mol
CO(g)+3H2(g)��H="+2a" kJ/mol
(2)B C
(3)��������ֹ������ijЩ����ʹ�����ж���Һ����(�����Һ̬��)
(4)���백���ʣ���������ѭ�����ã�������Ķ�����̼����������ϳ�����
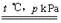 CO(g)+3H2(g)��H="+2a" kJ/mol
CO(g)+3H2(g)��H="+2a" kJ/mol (2)B C
(3)��������ֹ������ijЩ����ʹ�����ж���Һ����(�����Һ̬��)
(4)���백���ʣ���������ѭ�����ã�������Ķ�����̼����������ϳ�����
(1)0.5mol�����0.5molˮ������ȫ��Ӧ����һ����̼������(�ϳ���)��������a kJ��������Ӧ���1mol��ȫ��Ӧ����2aKJ�������Ȼ�ѧ����ʽ��CH4(g)+H2O(g)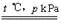 CO(g)+3H2(g)��H="+2a" kJ/mol��
CO(g)+3H2(g)��H="+2a" kJ/mol��
(2)�������ͨ������������Һ��������Һ�м����ᣬ���ɵĶ�����̼�л���HCl��A�������ö��ߵķе㲻ͬ���룬�������ѹ��ȴ��ʹCO2Һ�����Ӷ����룬B��ȷ���ð�ˮ���ն�����̼��ʣ�µ���������C��ȷ���������ͨ�뵽ʯ�ҽ���Ȼ��������չ��壬�����Ƚϸ��ӣ�������ʵ�֣�D����ѡBC��
(3)�����е�ijЩ�������Ǵ����ж��������ڿ�������ϳ���֮ǰ����Կ������о�����ʵ�����������У�������Һ��������ʹ��������������ٽ�ƽ�����ƣ���߷�Ӧ���ת���ʡ�
(4)�ϳɰ���ת���ʽϵͣ��������н϶��ԭ������ѭ��ʹ�ã�������Ķ�����̼���������Ʊ����ء�
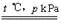 CO(g)+3H2(g)��H="+2a" kJ/mol��
CO(g)+3H2(g)��H="+2a" kJ/mol��(2)�������ͨ������������Һ��������Һ�м����ᣬ���ɵĶ�����̼�л���HCl��A�������ö��ߵķе㲻ͬ���룬�������ѹ��ȴ��ʹCO2Һ�����Ӷ����룬B��ȷ���ð�ˮ���ն�����̼��ʣ�µ���������C��ȷ���������ͨ�뵽ʯ�ҽ���Ȼ��������չ��壬�����Ƚϸ��ӣ�������ʵ�֣�D����ѡBC��
(3)�����е�ijЩ�������Ǵ����ж��������ڿ�������ϳ���֮ǰ����Կ������о�����ʵ�����������У�������Һ��������ʹ��������������ٽ�ƽ�����ƣ���߷�Ӧ���ת���ʡ�
(4)�ϳɰ���ת���ʽϵͣ��������н϶��ԭ������ѭ��ʹ�ã�������Ķ�����̼���������Ʊ����ء�

��ϰ��ϵ�д�
 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
�����Ŀ
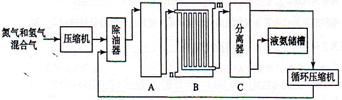
 CO2(g)+H2(g)
CO2(g)+H2(g)