��Ŀ����
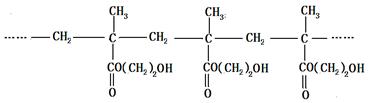
�������������۾����ϵľۺ���E�ǣ�
һ�ֺϳɾۺ���E��·�����£�
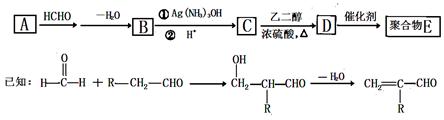
�ش��������⣺
��1��A��������Cu(OH)2����Һ��Ӧ����ש��ɫ������A�Ľṹ��ʽ�� ��
��2��D�к��еĹ���������Ϊ ��
��3��D��E�ķ�Ӧ������ ��Ӧ��
��4��C�ж���ͬ���칹�塣�������Һ���̼̼˫����ͬ���칹�干�� ��(������˳���칹)��д�����к˴Ź����������֮��Ϊ1��1��1��3��ͬ���칹��Ľṹ��ʽ ��
��5��д������ϩ�ϳ��Ҷ����Ļ�ѧ����ʽ�� ��

һ�ֺϳɾۺ���E��·�����£�

�ش��������⣺
��1��A��������Cu(OH)2����Һ��Ӧ����ש��ɫ������A�Ľṹ��ʽ�� ��
��2��D�к��еĹ���������Ϊ ��
��3��D��E�ķ�Ӧ������ ��Ӧ��
��4��C�ж���ͬ���칹�塣�������Һ���̼̼˫����ͬ���칹�干�� ��(������˳���칹)��д�����к˴Ź����������֮��Ϊ1��1��1��3��ͬ���칹��Ľṹ��ʽ ��
��5��д������ϩ�ϳ��Ҷ����Ļ�ѧ����ʽ�� ��
(15��)
��1��CH3CH2CHO(2��)
��2��̼̼˫�����������ǻ� (2��)
��3���Ӿ� (2��)
��4��5 (3��) HCOOCH=CHCH3 (2��)
��5��CH2=CH2+Br2 �� CH2BrCH2Br (2��) CH2BrCH2Br+2NaOH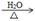 HOCH2CH2OH+2NaBr(2��)
HOCH2CH2OH+2NaBr(2��)
��1��CH3CH2CHO(2��)
��2��̼̼˫�����������ǻ� (2��)
��3���Ӿ� (2��)
��4��5 (3��) HCOOCH=CHCH3 (2��)
��5��CH2=CH2+Br2 �� CH2BrCH2Br (2��) CH2BrCH2Br+2NaOH
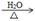 HOCH2CH2OH+2NaBr(2��)
HOCH2CH2OH+2NaBr(2��) �����������1����E�Ľṹ�����м�ȩ������ȩ��Ӧ����Ϣ���ɵ�A��CH3CH2CHO��
��2����E��֪C�к��ǻ���C�� D��D��E��֪C�к�˫����������������D�к��еĹ�����̼̼˫�����������ǻ���
��3����E�ijɷֿ�֪��D��E�ķ�Ӧ�����ǼӾ۷�Ӧ��
��4��5�֣��ֱ���HCOOCH2CH=CH2��HCOOCH=CHCH3��HCOO��CH3��CH=CH2��CH2="CHCOO" CH3��CH3COOCH=CH2.

��ϰ��ϵ�д�
 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д� ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д�
�����Ŀ
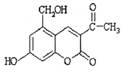



 ������������11��̼ԭ�Ӵ���ͬһƽ��
������������11��̼ԭ�Ӵ���ͬһƽ�� ��1 mol H2�����ӳɷ�Ӧ�ɵõ�3�ֲ�ͬ����
��1 mol H2�����ӳɷ�Ӧ�ɵõ�3�ֲ�ͬ���� �� �����й�˵����ȷ���ǣ� ��
�� �����й�˵����ȷ���ǣ� ��