��Ŀ����
��֪Ư����Ũ���ᷴӦ����������Ca(ClO)2 + 4HCl == CaCl2 + 2Cl2 + 2H2O��ijͬѧ��������װ���������ʵ�飺�ٴ��ԲⶨijƯ������Ч�ɷ�Ca(ClO)2�ĺ�������̽�����﴿����������Ư���ԣ���ʪ��������Ư���ԣ���Ư��������HClO�����á��ش��������⣺

��1��д����ҵ����ȡƯ�۵Ļ�ѧ����ʽ_________________________________
��2��������з�Ӧ����ת�Ƶķ������Ŀ����ָ����Ӧ�е��������ͻ�ԭ����
Ca(ClO)2 + 4HCl == CaCl2 + 2Cl2 + 2H2O
������_______________��ԭ��_________________
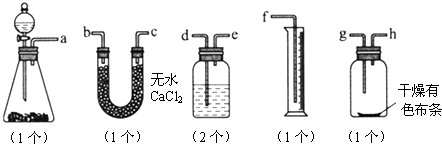
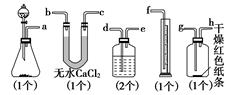
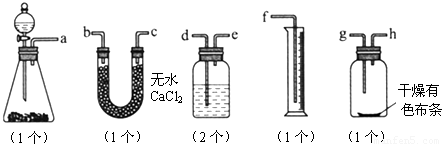
��3������ʵ��װ��B��Ӧ�ŵ��Լ���_____________��������_____________
��4��ijͬѧ��ij��ʵ��ʱ��ȡƯ����Ʒmg��ʵ��������ȡ��Ͳ��ˮ�����ΪVmL������ʵ��������DZ�״���������Ư������Ч�ɷֵĺ���____________________________���ú���ĸ�İٷ�����ʾ��
��5��ϸ�ĵ���ͬѧ˼�����ָ�ʵ���̽��ʵ����ۣ���һ�����ԵIJ��㣬�ò���֮���ǣ�����Ϊû�в��㣬�ÿղ���д��__________________________________________________��
��1��2Ca(OH)2 +2 Cl 2== CaCl2 + Ca(ClO)2 + 2H2O
��2������ת�ƣ��ԡ�Ca(ClO)2 HCl
��3��Ũ���ᣬ����������
��4��![]() %��
%��
��5������ʪ����ɫ������ɫ��������˵���Ǵ������Ư�����á�

 ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д� ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�