��Ŀ����
����NH4I����һ�����ΪV L������ܱ������У�������һ���¶Ⱥ����·������з�Ӧ��NH4I��s��NH3��g����HI��g����2HI��g��
H2��g����I2��g����ƽ��ʱ��������������干Ϊ5 mol������HIΪ1��5 mol��������˵����ȷ���� ��������
A��ƽ��ʱ����Ϊ2��5 mol
B��ƽ��ʱHI�ķֽ���Ϊ20%
C������ʼʱ�������NH4I��������һ������ƽ��ʱ���干Ϊ10 mol
D�������������䣬�������������С��L�����´�ƽ��ʱH2��Ũ����ԭƽ���2��
A
����:��

��ϰ��ϵ�д�
�����Ŀ
����NH4I����һ�����ΪVL������ܱ������У�������һ���¶Ⱥ����·������з�Ӧ��NH4I(s) NH3(g)��HI(g)��2HI(g)
NH3(g)��HI(g)��2HI(g) H2(g)��I2(g)��ƽ��ʱ��������������干Ϊ5mol������HIΪ1.5mol��������˵����ȷ���ǣ� ��
H2(g)��I2(g)��ƽ��ʱ��������������干Ϊ5mol������HIΪ1.5mol��������˵����ȷ���ǣ� ��
| A��ƽ��ʱ����Ϊ2.5mol |
| B��ƽ��ʱHI�ķֽ���Ϊ20% |
| C������ʼʱ�������NH4I��������һ������ƽ��ʱ���干Ϊ10 mol |
| D��������������������������С��V/2 L�����´ﵽƽ��ʱH2��Ũ����ԭƽ���2�� |
 NH3(g)��HI(g)��2HI(g)
NH3(g)��HI(g)��2HI(g)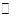 NH3��g����HI��g����2HI��g��
NH3��g����HI��g����2HI��g��