��Ŀ����
ͨ����ʵ��Ĺ۲졢�����������ó���ȷ�Ľ����ǻ�ѧѧϰ����Ҫ����֮һ��������ʵ����ʵ������Ľ�����ȷ����
| A��KI������Һ��ͨ��Cl2����Һ����ԭ���ǵ�������Cl2������ɫ��Ӧ |
| B��Ũ�����ڹ��������±��������Ũ����ȶ���������ɫ����������Ũ���� |
| C��ij��Һ�м��������ữ���Ȼ�����Һ���а�ɫ��������˵������Һ�к���SO42- |
| D�������£�Ũ����ɴ��������ʻ�����������˵����������������Ũ�����Ӧ |
B
��������ǿ�����ԣ���KI�������ɵ��ʵ⣬������������ɫ��Ũ����ȶ������ֽ�����NO��NO2��H2O��NO2����ˮʹ����ʻ�ɫ����Һ�м��������ữ���Ȼ�����Һ���а�ɫ�������ɣ�����������AgCl��BaSO4������������������Ũ�����з����ۻ����ۻ����ڻ�ѧ�仯������B��

��ϰ��ϵ�д�
�����Ŀ
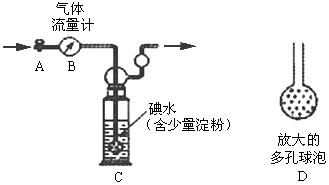
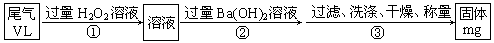
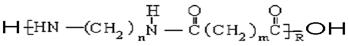 �ĵ���Ϊ___________
�ĵ���Ϊ___________ 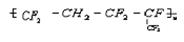 �ϳ����ĵ���Ϊ________________________
�ϳ����ĵ���Ϊ________________________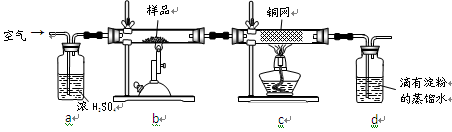
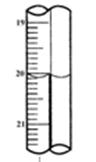
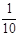 ������ƿ�У���0��05mol/L������Һ���еζ���������Ϊ0��10mL��ĩ��������ͼ��ʾ��
������ƿ�У���0��05mol/L������Һ���еζ���������Ϊ0��10mL��ĩ��������ͼ��ʾ��