��Ŀ����
��.ʵ������ͨ������ڵ�����ˮ�еμӱ����Ȼ�����Һ��ȡFe(OH)3���塣�ش���������
(1)Fe(OH)3�����________ɫ����ȡ���Ļ�ѧ����ʽΪ________________________��
(2)ͨ��____________ʵ�������֤����������Һ���ǽ��塣
(3)�������������ܱȽ��ȶ����ڵ�ԭ����________��
A������ֱ��С��1 nm B������������� C�������������˶� D�������ܹ�ͨ����ֽ
��.
(4)��ʲô�������Գ�ȥ���۽����к��е�NaCl���ʣ���ʵ������ɸ�ʵ�����ʹ���ձ�������������Ҫʹ��ʲôʵ����Ʒ(ҩƷ����)��
____________________________
(5)�����֤(4)�е��۽����Ѿ���������(д�������������)��
____________________________
(1)Fe(OH)3�����________ɫ����ȡ���Ļ�ѧ����ʽΪ________________________��
(2)ͨ��____________ʵ�������֤����������Һ���ǽ��塣
(3)�������������ܱȽ��ȶ����ڵ�ԭ����________��
A������ֱ��С��1 nm B������������� C�������������˶� D�������ܹ�ͨ����ֽ
��.
(4)��ʲô�������Գ�ȥ���۽����к��е�NaCl���ʣ���ʵ������ɸ�ʵ�����ʹ���ձ�������������Ҫʹ��ʲôʵ����Ʒ(ҩƷ����)��
____________________________
(5)�����֤(4)�е��۽����Ѿ���������(д�������������)��
____________________________
(1)��֣�FeCl3��3H2O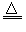 Fe(OH)3(����)��3HCl
Fe(OH)3(����)��3HCl
(2)�����ЧӦ
(3)BC
(4)��������Ĥ��ϸ��
(5)ȡ�ձ������һ������Һ������һ֧�ྻ���Թ��У��μӼ��������ữ����������Һ�������û�г���������˵�������Ѿ�����������(������������)
 Fe(OH)3(����)��3HCl
Fe(OH)3(����)��3HCl(2)�����ЧӦ
(3)BC
(4)��������Ĥ��ϸ��
(5)ȡ�ձ������һ������Һ������һ֧�ྻ���Թ��У��μӼ��������ữ����������Һ�������û�г���������˵�������Ѿ�����������(������������)

��ϰ��ϵ�д�
 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�
�����Ŀ